Bridge Report:(4597)Solasia Pharma Second Quarter of the Fiscal Year Ending December 2024
 President Yoshihiro Arai | Solasia Pharma K.K. (4597) |
 |
Company Information
Market | TSE Growth Market |
Industry | Pharmaceutical products (manufacturing) |
President | Yoshihiro Arai |
HQ Address | 4F SUMITOMO FUDOSAN SHIBA-KOEN TOWER, 2-11-1, Shiba-koen, Minato-ku, Tokyo |
Year-end | December |
Homepage | https://solasia.co.jp/en/ |
Stock Information
Share Price | Shares Outstanding (Term end) | Total Market cap | ROE Act. | Trading Unit | |
¥51 | 199,944,010 shares | ¥10,197 million | -49.0% | 100 shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est. | BPS Act. | PBR Act. |
¥0.00 | - | ¥-4.01 ~ -2.76 | - | ¥9.85 | 5.2x |
*The share price is the closing price on August 29. The number of outstanding shares, DPS, EPS, and BPS are from the brief report on the financial results for the second quarter of the fiscal year ending December 2024. ROE is from the results in the previous fiscal year.
Earnings Trends
Fiscal Year | Sales | Operating profit | Ordinary profit | Net profit | EPS | DPS |
December 2020 | 454 | -4,116 | -4,159 | -4,127 | -35.16 | 0.00 |
December 2021 | 559 | -2,419 | -2,442 | -2,478 | -19.04 | 0.00 |
December 2022 | 1,092 | -2,470 | -2,492 | -2,548 | -16.77 | 0.00 |
December 2023 | 617 | -1,139 | -1,135 | -1,112 | -6.62 | 0.00 |
December 2024 Est. | 1,250 ~ 1,500 | -800 ~ -550 | -800 ~ -550 | -800 ~ -550 | -4.01 ~ -2.76 | 0.00 |
* The forecast is from the company. IFRS application. Net profit is profit attributable to owners of the parent. Hereinafter the same shall apply.
This report outlines Solasia Pharma’s pipeline development status, financial results for the second quarter of the fiscal year ending December 2024, and future outlook.
Table of Contents
Key Points
1. Company Overview
2. Second Quarter of the Fiscal Year Ending December 2024 Earnings Results
3. Fiscal Year Ending December 2024 Earnings Forecasts and Future Goals
4. Conclusions
<Reference: Regarding Corporate Governance>
Key Points
- The sales revenue in the second quarter of the fiscal year ending December 2024 was 72 million yen, down 457 million yen year on year. It includes revenue from sale of “DARVIAS® (SP-02).” The sales partners built up inventory of products manufactured at the old facility during the previous fiscal year to prevent shortages in the market during the period of shift to products manufactured at the new facility, resulting in smaller shipments of products manufactured at the new facility.
- R&D expenses increased 31 million yen year on year to 223 million yen. This was mainly due to investment in the change of manufacturing facilities to reduce product cost, consideration of expansion of indications for “DARVIAS® (SP-02)” and clinical development in China, animal experiments for SP-04, and investment in candidates for products to be developed. SG&A expenses decreased 169 million yen year on year to 390 million yen. As a result, operating loss was 611 million yen year on year, down 149 million yen year on year.
- For the fiscal year ending December 2024, Sales revenue is projected to be at least 1,250 million yen, which is the sum of 500 million yen as revenues from sale of “Sancuso® (SP-01),” “Episil® (SP-03),” and “DARVIAS® (SP-02)” and 750 million yen as lump-sum revenues from the conclusion of the contract for licensing out “DARVIAS® (SP-02)” in China. Although the impact of inventory liquidation and other factors related to the relocation of manufacturing sites for “Sancuso® (SP-01)” and “Episil® (SP-03)” will gradually weaken, it is expected to continue for the time being. Under the assumption that the company will earn all short-term milestone income from the contract for licensing out “DARVIAS® (SP-02)” in China in 2024, an additional lump-sum payment from the contract is expected to amount to 250 million yen, so the upper limit is assumed to be 1.5 billion yen. The company is forecast to post an operating loss and other losses ranging from 550 million yen to 800 million yen.
- Once it appeared that the development of "SP-05: arfolitixorin" had failed, it became clear that a major reason for the failure was that neither dosage nor administration was optimal. The company believes that the optimized dosage and administration of "SP-05: arfolitixorin" has a high potential to deliver better results and is currently considering when to participate in the clinical development program led by Isofol.
- It is said that there are more than 150,000 patients with colorectal cancer in Japan each year, making it one of the most common cancers in Japan. Currently, most of the existing competitive products for "SP-05: arfolitixorin" are generic drugs, and the Japanese market scale is expected to be approximately 30 billion yen. New treatments have not been developed as standard treatments for colorectal cancer for over 10 years. If "SP-05: arfolitixorin" were to replace existing standard treatments for colorectal cancer, the survival rate of colorectal cancer patients would increase significantly, and the company believes that the success of the development would be highly valued.
- Sales of "Sancuso® (SP-01)" and "Episil® (SP-03)" have been smaller than expected due to the transfer of manufacturing sites. Still, the high recognition of the efficacy and usefulness of these products has not changed. Although there are issues to be addressed, such as the situation in the medical field and relationships with sales partners, we will keep a close eye on the company's performance from the second half of this year onward, when the impact of the transfer of manufacturing sites will fade away. Regarding the highly anticipated licensing-out of the Chinese rights of "DARVIAS® (SP-02)," negotiations with a potential licensee are said to be proceeding at a considerable rate. We look forward to the release of a decision on the licensee. At the same time, we would like to focus on the progress of the clinical development program led by Isofol for "arfolitixorin SP-05" as well.
1. Company Overview
As a specialty pharma* specializing in oncology, Solasia Pharma develops and sells medicines for cancer treatment and supportive care, etc. in Asia, mainly Japan and China, each of which has a promising market.
Its significant strengths and features are the development staff with abundant practical experience led by CEO Arai, high rate of successful development, the stable business foundation, feasibility of business model, and so on.
*Specialty Pharm A new drug developing enterprise possessing research and development capabilities which has a certain standard in its field of expertise, both domestically and internationally.
1-1 Corporate History
Its predecessor is Japan Bridge Inc., which was established as a foothold for preparing for the business of developing pharmaceutical products in the U.S. in December 2006 jointly by ITOCHU Corporation and MPM Capital, a U.S. venture capital specializing in bio business.
In May 2008, the company introduced the exclusive right to develop and sell the first product “Sancuso® (SP-01)” in Japan, Taiwan, Singapore, Malaysia, and China, including Hong Kong and Macau.
In September 2008, the company was renamed Solasia Pharma K.K.
Then, the company introduced the exclusive right to develop and sell “DARVIAS® (SP-02)” in the Asia-Pacific region (March 2011), introduced the exclusive right to develop and sell it around the world, including the U.S. and Europe (July 2014), and introduced the exclusive right to develop and sell “Episil® (SP-03)” in Japan and China (March 2015), to enrich pipelines. The company also provided Kyowa Kirin Co., Ltd. with the exclusive license to develop and sell “Sancuso® (SP-01)” in Taiwan, Hong Kong, and so on. (February 2010) and provided Lee's Pharmaceutical (HK) Limited with the exclusive license to sell “Sancuso® (SP-01)” at the time of the conclusion of the contract in China (excluding Beijing, Shanghai, Guangzhou, Hong Kong, and Macau). All these paved the way for monetization.
In 2016, the company applied for the approval for manufacturing and sales of medical apparatus for “Episil® (SP-03)” in China and Japan, and provided Meiji Seika Pharma Co., Ltd. with the exclusive distributorship in Japan and provided Lee’s Pharmaceutical (HK) Limited with the exclusive distributorship at the time of the conclusion of the contract in China (excluding Beijing, Shanghai, and Guangzhou).
As the company was expected to grow as a pharmaceutical company specializing in cancer, it was listed in Mothers of Tokyo Stock Exchange in March 2017.
In November 2017, the company acquired the exclusive right to develop and sell for "PledOx® (SP-04)" in Japan, China, South Korea, Taiwan, Hong Kong, and Macau. In May 2018, “Episil® (SP-03)” was released in Japan, as the first product released by the company. Next, Sancuso® (SP-01) and Episil® (SP-03) were launched in China in 2019, followed by Episil® (SP-03) in South Korea in 2020, and DARVIAS® (SP-02) in Japan in August 2022, thus moving from the "development" stage to the "sales and commercialization" stage.
In April 2022, the company got listed on the Growth Market of the Tokyo Stock Exchange in accordance with market reorganization.
1-2 Corporate Philosophy・Management Philosophy
The company’s name, SOLASIA, is a coined word combining Sol (the Sun in Latin) and Asia (Asian counties). It represents the company’s mission which is to be the Sun brightening the future of various people facing many challenges of cancer in Japan and other Asian countries.
The management philosophy adopts the following mission, vision, and values.
Role to Fulfill (Mission) | *Better medicine for a brighter tomorrow |
Ideal Situation (Vision) | *To be recognized domestically and overseas and gain a high level of trust from all stakeholders. |
*To be recognized as a specialty pharma developing innovative medicine, where each employee possesses passion, ambition, and a sense of morality, strives to better themselves, maintains a high level of expertise, and continuously endeavors for new value and creation for the future. | |
*To meet the needs of people (medical practitioners and patients) who need our products and contribute to them. | |
Shared Values (Value) | *Create value for patients. |
*Have high ethical standards. | |
*Trust and respect each other. | |
*Work as a team. |
In addition, the following two points are listed as management policy.
① For the time being, we will continue the in-licensing of new products in cancer and rare disease field where major pharmaceutical companies do not emphasize from a performance-based approach and contribute to patients without adequate medication. |
② Through the commercialization of products, we will promptly establish the financial stability needed to realize our management philosophy, and secure independence. |
The company will focus on developing new drugs to solve unmet medical needs (medical needs for diseases for which no treatment has been developed), which is a niche market but has many troubled patients. As research and development is proceeding, they will have to rely on financing CF now, but they plan to make operating CF positive soon and build a strong basis to achieve continuous growth.
1-3 Environment Surrounding Solasia Pharma
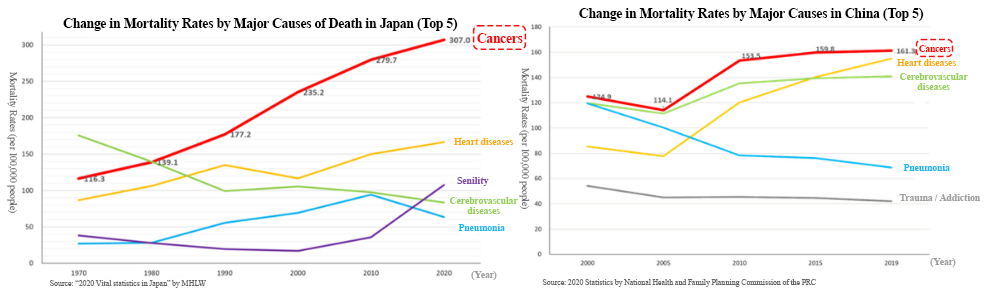
According to “Vital Statistics, 2020” published by the Ministry of Health, Labour and Welfare, in 2020, the leading cause of death was malignant neoplasm (cancer), 307.0 per 100,000 people. In 1981, cancer overtook cerebrovascular diseases, the former number one cause of death, with the mortality rates from cancer being 142.0 and that from cerebrovascular diseases being 134.3. Since then, cancer has been the leading cause of death for the 30 consecutive years and keeps going up every year.
As it is said that the incidence rate of cancer is rising due to aging and changes in lifestyles including diet, the number of patients and deaths regarding cancer is rising in China as well.
(Source: Solasia Pharma)
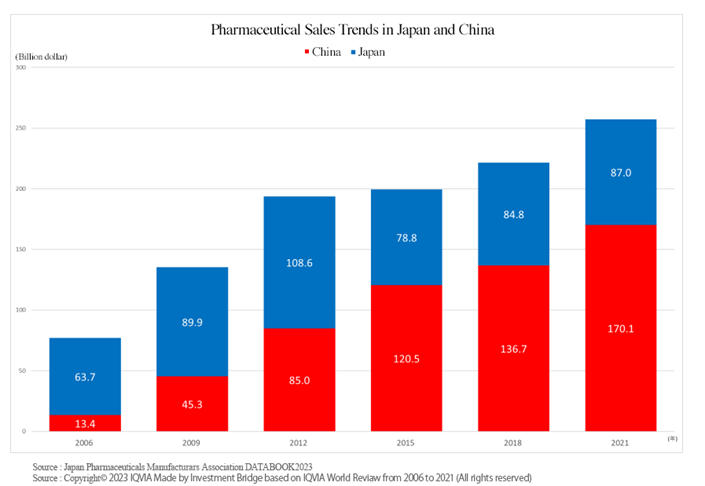
Amid such situation, the sales of the world’s pharmaceutical market in 2021 were 1,439.5 billion US dollars (approximately 190 trillion yen). The U.S. has the largest pharmaceutical market, followed by China, which overtook Japan in 2013, and Japan, which has the third largest market.
In the future, it is said that the market in China will expand to the point where it will share the top position with the U.S.
The total market size of China, the second biggest country, and Japan, the third biggest country, is 257.1 billion dollars (about 34 trillion yen). For the time being, this huge market will be the company’s main target.
(Source: Solasia Pharma)
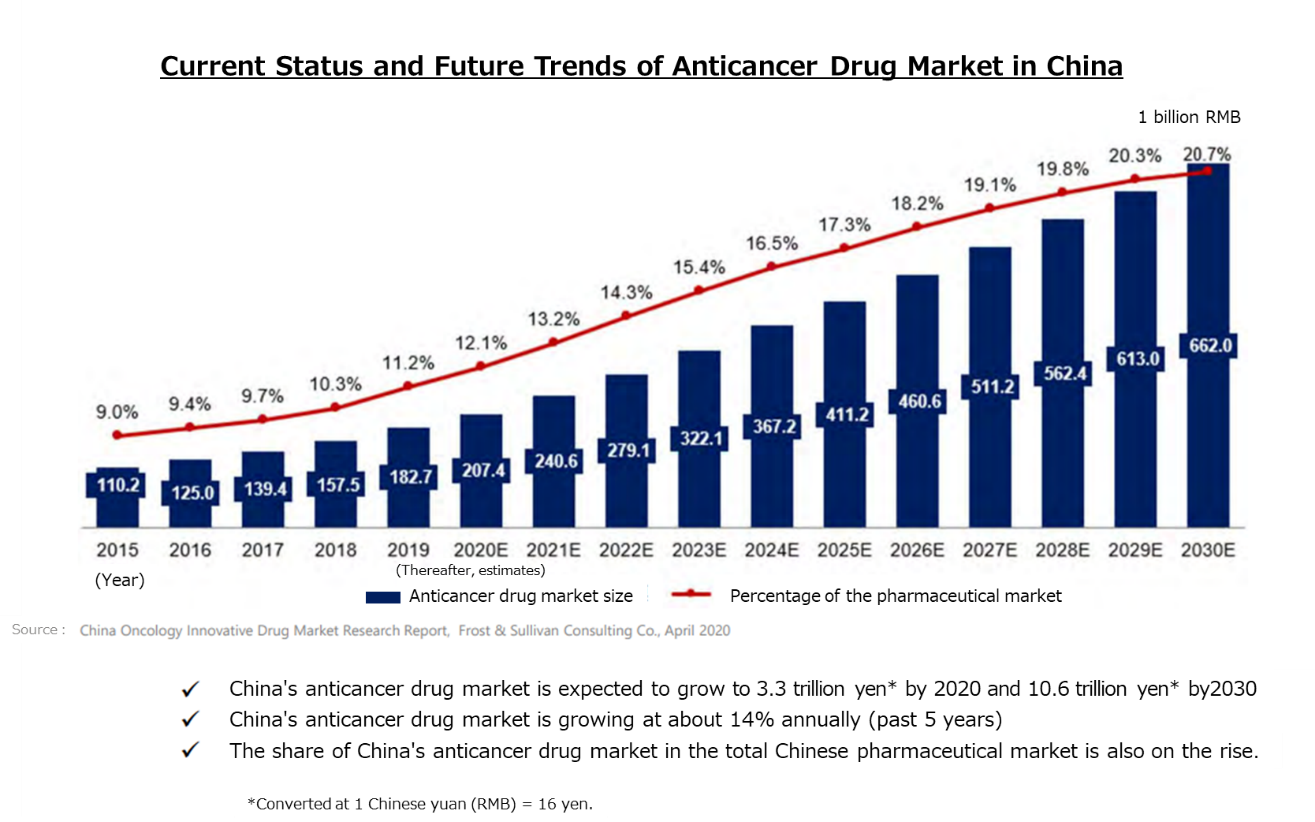
In addition, the anticancer drug market in China is over 3 trillion yen, accounting for more than 10% of the total pharmaceutical market, and it has grown at a CAGR of approximately 14% over the past five years.
(Source: Solasia Pharma)
As the mortality rates from cancer increases as shown above, expectations for “new anticancer drug” and “cancer supportive care” are growing all over the world.
(New anti-cancer drug)
In cancer treatment provided using anticancer drug, it is said that a majority of hospitals use the polytherapy which uses multiple anticancer more than the monotherapy which uses a single anticancer drug.
In addition, although it depends on cancer types, there is significant risk of relapses. Besides, in case of intractable cancers, it is difficult to cure such cancers only with a single treatment method, which means that a single medicine is not always an absolute cure, and therefore, other therapeutic medications will hardly be direct “competing products.” Molecular targeted drugs and immunotherapy have also attracted attention in recent years, however chemotherapeutic agents still hold an important position for treatment of many cancer types. Standard therapy involves a regimen containing a cytotoxic anticancer drug, for which a high medical demand is expected in the future as well.
(Cancer supportive care)
Anticancer drugs are potent medicine that attacks cancer cells, and side effects are inevitable.
If the side effects on patients cannot be controlled, anticancer therapy through drug administration must be stopped, which has a risk of resulting in cancer progression.
As a result, expectations for drugs and medical devices which control such side effects are increasing in order to avoid treatment discontinuation and complete cancer treatment. In addition, while therapeutic drugs for cancer must be approved for each cancer type, supportive care can be provided to a wide range of patients regardless of cancer types, which means that there will be strong needs and markets.
In summary, needs for cancer treatment in Japan and China are growing and there are great expectations for new anticancer drugs and cancer supportive care. Solasia Pharma is establishing business model and business strategy to incorporate such needs and boost earnings.
1-4 Business Description
(1) Business Model
Before the launch of new medicines, it is usual to go through the processes spanning from “basic research” to “pharmaceutical research,” “nonclinical development (trials conducted using animals to examine medicinal and pharmacological action, in-vivo pharmacokinetic properties, adverse effects, etc.),” and “clinical development (scientific trials carried out to examine the effects of pharmaceuticals and treatment techniques on human beings), obtain approval from the authorities, and then conduct “manufacture” and “sales, marketing, and post-marketing surveillance.”
Although major pharmaceutical companies are propelling outsourcing to CROs at the stage of clinical development to make considerable amounts of R&D expenses variable, they basically perform all of the above-mentioned processes internally.
Such a system has supported high profitability of pharmaceutical companies. The life science field, however, is currently advancing and becoming complicated and diverse at a rapid rate, and there is an increasing possibility that each company’s unique drug discovery technology quickly becomes obsolete.
In addition, there are a myriad of cases where practical application of new drugs is given up before clinical development, regardless of costs and time spent from the stage of basic research, and therefore new drug is not established in the end. In other words, pharmaceutical development is always facing high risks.
Accordingly, Solasia Pharma does not conduct the processes from basic research to nonclinical development on its own which has high failure rate. By in-licensing promising pharmaceuticals that are still under development from outside companies, it embarks on development starting from clinical development. It utilizes its strength and reduces risk by focusing management resources on the business activities after the development stage. At the moment, it plans not to do manufacturing due to the large cost burden.
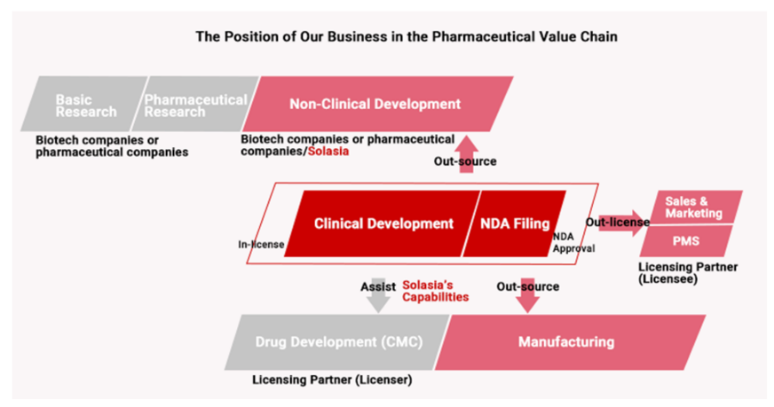
(Source: Solasia Pharma)
Regarding the sales and marketing structure, the company has set up a system that takes into account the balance between high profitability and risk control.
In general, pharmaceutical companies hold gross profit margins to high standards, which is considered to be attained by their in-house manufacture and sales activities.
| Sales Revenue | Gross Profit | Gross Profit Margin |
Astellas Pharma | 1,603,672 | 1,311,187 | 81.8% |
Daiichi Sankyo | 1,601,688 | 1,186,366 | 74.1% |
*Unit: million yen. The values are the results from fiscal year ended March 2024.
On the other hand, coverage of sales territories (e.g., to cover all over Japan) is required for pharmaceuticals, and therefore, a rise in fixed costs is inevitable for establishing a company’s own sales network. Accordingly, Solasia Pharma uses “licensing-out model” (sales rights are granted to other companies for pharmaceuticals that have completed clinical development).
(Self-selling model)
The current major licensing-out partners are the following four companies.
Meiji Seika Pharma Co., Ltd. | *A pharmaceutical company of the Meiji Group. It is a specialty pharma in the fields of cancer, infections, and the central nervous system and has yielded sales results of multifarious products in the cancer field. *Japanese partner with the rights of “Episil® (SP-03)” |
Nippon Kayaku Co., Ltd. | *Founded in 1916. The company specializes in cancer-related products in the pharmaceutical business, handles everything from new drugs to biosimilars and generics, and provides medical institutions with highly reliable information necessary for anticancer drugs. *Japanese partner with the rights of DARVIAS® (SP-02) |
Lee’s Pharmaceutical (HK) Limited | *A Chinese pharmaceutical company listed on the Hong Kong market. It sells multiple pharmaceutical products in fields including the cancer field across China through about 30 bases. *Chinese partner with the rights of “Sancuso® (SP-01)” (excluding Beijing, Shanghai, and Guangzhou) *Chinese partner with the rights of “Episil® (SP-03)” (excluding Beijing, Shanghai, and Guangzhou) |
Maruho Co., Ltd. | *A pharmaceutical company that was founded in 1915 and engages in the research, development, production, and sale of pharmaceutical products, etc. It is especially excellent in the dermatological field. |
Solasia Pharma plans to create licensing-out partnerships with a focus on mid-sized pharmaceutical companies which it can fall in line easily and forge win-win relationships.
(2) Marketing structure in China
The company, aiming to develop the vast Chinese pharmaceutical market, has entrusted the sales of “Sancuso® (SP-01)” and “Episil® (SP-03)” across China to Lee’s Pharmaceutical (HK) Limited.
Point: Highly regarded by Chinese medical community
The company abandoned the development of a system for selling products by themselves in China, but their basic conditions for cultivating the huge market in China have not been changed, as mentioned in Section 1-3 “Environment Surrounding Solasia Pharma.”
The judgement and decision of influential physicians greatly affect the outcome of the use and distribution of new medicines, and China is no exception.
Under these circumstances, “Sancuso® (SP-01)” is already recommended as one of the standard treatments for nausea and vomiting in the Chinese version of the NCCN guidelines for cancer treatment, which is referenced in the clinical sites.
In addition, at Chinese Society of Clinical Oncology (CSCO), prominent clinicians who are leading the field of cancer treatment in China highly valued “Sancuso® (SP-01)” for its feature of easily suppressing nausea and vomiting in the entire chemotherapy process. In response to this, “Sancuso® (SP-01)” is listed as a standard antiemetic treatment option for cancer treatment in the first guideline for proper use of antiemetics issued by CSCO.
The company is receiving such a high rating because of the superior efficacy of “Sancuso® (SP-01)”. But it is obvious that the strong relationship with the Chinese clinical network that the management team had been building since their times with Roche is also playing a key role, and it is a major advantage of the company that other bio-ventures do not have.
(3) Products/Development Pipeline
The company currently has a total of five products and those in the pipeline: three products for sale (approved products) and two products under development, in line with the aforementioned management policy. (As of August, 2024)
(Source: Solasia Pharma)
【Products for sale (Approved products)】
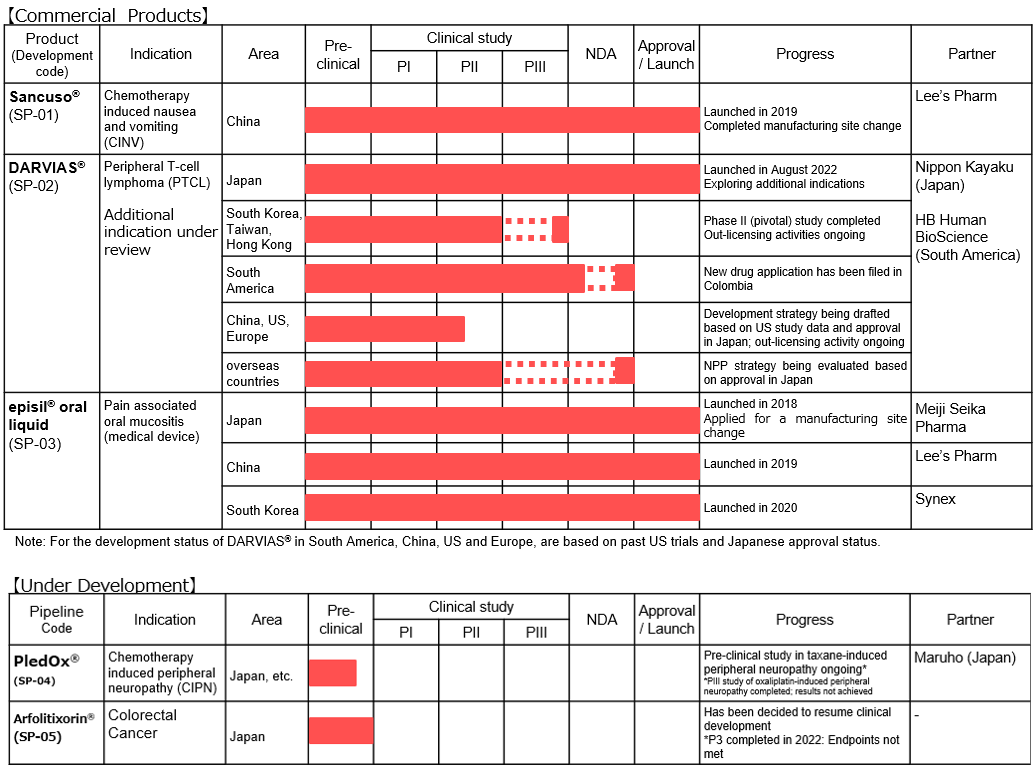
“SP-01: Transdermal Delivery System Sancuso®” (Sales name in Chin 善可舒®)
(※)CSCO(Chinese Society of Clinical Oncology) : The most prominent and largest academic conference related to cancer in China
(Overview of indications)
Nausea and vomiting are widely known as typical side effects caused by anticancer drug.
Administration of anticancer drug damage cells called Chromaffin cells in the small intestine.
The damaged Chromaffin cells produce serotonin, a neurotransmitter, which is taken in by the 5-HT3 receptors in the peripheral vagus nerve. This stimulus is transmitted through the peripheral vagus nerve to the medulla oblongata via the chemoreceptor trigger zone (CTZ) in the area postrema of the fourth ventricle of the brain, stimulating the vomiting center which gives living organisms commands to develop nausea and vomiting, and then symptoms of nausea and vomiting appear.
It is necessary to disrupt the stimuli generated by serotonin to the 5-HT3 receptors in order to control nausea and vomiting. There are a variety of “5-HT3 receptor antagonists” which are drugs used for the above purpose, and one of the representative agents is Granisetron.
(Overview of “Sancuso® (SP-01)”)
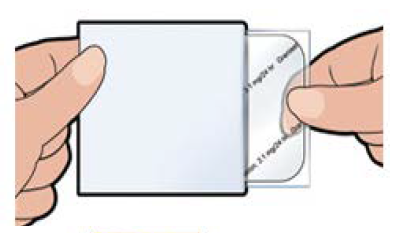
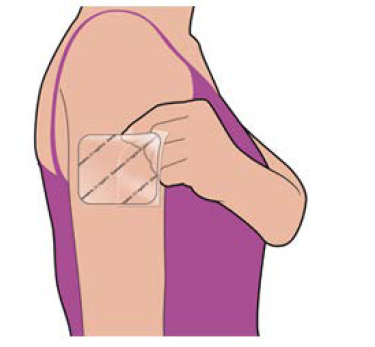
“Sancuso® (SP-01)” is a transdermal 5-HT3 receptor antagonist containing Granisetron and is the world’s only patch-type antagonist.
 |
 |
 |
*Chinese package of Sancuso® |
|
|
(Source: Solasia Pharma)
Anticancer drugs are administered over 5 days in most cases, but injections and oral antiemetic agents are effective only for about 1 to 2 days and must be injected multiple times within the anticancer drug administration period. On the other hand, “Sancuso® (SP-01)” maintains the concentration level of Granisetron in blood on a stable basis for 5 days. Therefore, once a patch of “Sancuso® (SP-01)” is attached, there is no need to add antiemetics, which enables cancer treatment not through hospitalization but through outpatient care and contributes significantly to the improvement of patients’ quality of life.
Another advantage is that transdermal type drugs can be administered even to patients who are facing difficulty in taking oral medicines due to various symptoms including nausea, vomiting, and stomatitis. Earning reputation for the above-mentioned advantages, “Sancuso® (SP-01)” is recommended for prescription in the American NCCN clinical practice guidelines and the Chinese clinical practice guidelines.
(Current situation of development and commercialization)
◎ China
The company finalized their application for approval in June 2014, and obtained approval in July 2018, along with permission to import drug license. It received milestone payments in the third quarter of fiscal year December 2018, and the sales revenue was recorded.
After that, they established a process for manufacturing commercially-available products, and sales started in 2019. Since August 1, 2022, Lee’s Pharma, which is the licensee, has engaged in sales activities throughout China.
In order to reduce product manufacturing costs, the company applied to the Chinese authorities for a manufacturing facility change, which was approved at the end of 2023.
Meanwhile, in the first half of 2024, shipment volume declined because Lee's, the company's sales partner, built up inventory of products from the old manufacturing facility to prevent shortages in the market during the period when products are replaced by those manufactured at the new manufacturing facility.
Although sales are currently more sluggish than expected, the company has received high praise from the medical community as follows.
Evaluation comments from major Chinese clinicians
On March 16, 2019, the company held (co-sponsored) the “ Sancuso® China national launching meeting” in Shanghai.
The chairman of Chinese Society of Clinical Oncology (CSCO), Professor Li Jin, and the vice chairman, Professor Qin Shukui and Professor Ma Jun were chairmen of the meeting, a total of approximately 200 oncologists from all over China attended the meeting. At that meeting, Chinese key opinion leaders made remarks on “SP-01: Sancuso®” as follows.
Professor Qin Shukui (Vice Chairman of CSCO)
“Without any anti-emetic measures, 70%-80% of chemotherapy patients would experience CINV which would severely affect their quality of life. Often, patients will have to be treated with reduced dosage or even withdrawn from chemotherapy, with negative impacts on the treatment outcomes. The traditional CINV prevention methods are mainly short-term intravenous injection, which due to great fluctuation in blood concentration, requires repeated administration which is inconvenient for patients. With unique transdermal system, Sancuso® gradually releases granisetron into blood every day for up to 7 days. With one patch per one chemotherapy cycle, it is a new non-invasive treatment choice for chemotherapy patients.”
Professor Ma Jun (Vice Chairman of CSCO)
“The emetic risk in patients receiving HEC and MEC chemotherapy will continue for 2-3 days after last dose of chemotherapy. For multi-day chemotherapy, there is an overlap between acute and delayed vomiting, which requires more stable and long-lasting drug. Sancuso® covers different emetic stages including expected, acute and delayed nausea and vomiting. The 7 days stable efficacy makes the entire process CINV management possible and allows patients to feel at ease throughout the entire chemotherapy cycle.”
Professor Li Jin (Chairman of CSCO)
“The successful launching of Sancuso provides a long-lasting, stable and non-invasive new choice for the prevention of nausea and vomiting in Chinese chemotherapy patients. As a new choice for the prevention and treatment of chemotherapy related vomiting, with one patch, which is simple and easy, it makes CINV entire process management more convenient, it helps to standardize clinical treatment of CINV and further improves the treatment rate of CINV.”
CSCO’s first guideline for proper use of antiemetics was issued.
In June 2019, three months after Sancuso® (SP-01) was launched, CSCO issued the first guideline for proper use of antiemetics, and it was listed as a standard antiemetic treatment option for cancer treatment.
Prof. Qin Shukui, deputy director of CSCO and Guideline team leader, said, “This guideline recommends Sancuso® for an antiemetic treatment against highly and moderately emetogenic chemotherapy, providing a non-invasive and tolerable treatment option to cancer patients.”
The company plans to grow 6% on the basis of quantity and aims to increase share in China’s 5-HT3 RA antiemetic market, which is said to be 80 billion yen or more.
◎ Future sales strategy
In China, Lee's Pharma plans to focus on expanding sales by targeting indications beyond cancer chemotherapy, including radiotherapy and anesthesia-induced nausea and vomiting.
“SP-02:novel chemotherapeutic agent DARVIAS® ”
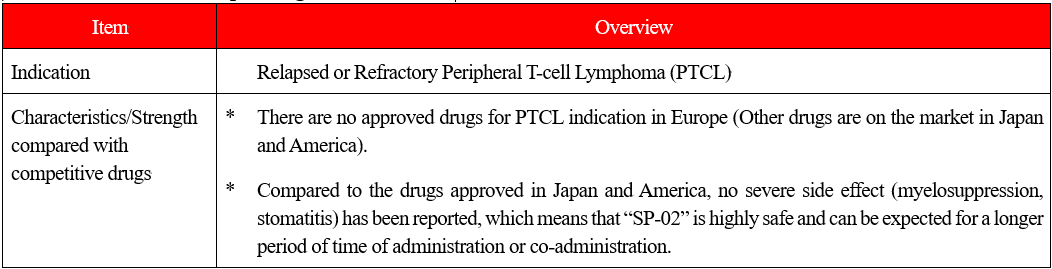
(Overview of indications)
Malignant lymphoma is one type of hematologic cancer where lymphocytes in white blood cells become cancerous.
The types of lymphocytes include B cells, T cells, and NK cells, and when these cells become cancerous and continues uncontrolled growth, malignant lymphoma develops.
Peripheral T-cell lymphoma (PTCL) is one kind of malignant lymphoma which arises from T cells in lymphocytes and is categorized into the “intermediate-grade lymphoma” where the disease progresses monthly, and it is said to account for 10-15% of the intermediate-grade lymphoma. The five-year survival rate from malignant lymphoma is lower than that from B-cell lymphoma, with the ratio being around 25%.
Estimated number of PTCL patients (Japan): Approximately 4,000/year*
(Development status)
The development of “DARVIAS® (SP-02)” started aiming for recurring/intractable peripheral T-cell lymphoma (PTCL) indication as mentioned above.
The early second phase clinical trials in the U.S. were completed in April 2012 and have shown certain efficacy in Caucasians.
In the first phase clinical trial completed in April 2015 in Japan and Korea, safety and tolerability of the drug were confirmed, with certain efficacy in Asians suggested.
In addition, the international phase II study, which was started in Japan, South Korea, Taiwan, and Hong Kong in was completed in September 2019.
As originally planned, the trial results were announced in June 2020 following statistical analysis. In addition to meeting the requirements for the primary endpoint, which is antitumor effect, no safety concerns were noted regarding secondary endpoints either. As positive results were achieved, the company applied for the approval for manufacturing and sales in Japan in June 2021 for the first time in the world. On June 20, 2022, the company obtained manufacturing and marketing approvals from the Ministry of Health, Labour and Welfare for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL).
It is known that malignant lymphoma often relapses. Accordingly, Solasia Pharma believes that multiple medicines with different mechanisms of action are necessary, and the market scale is significant.
In addition to seeking to expand the use of the drug by verifying and proposing synergistic effects when combined with other drugs for peripheral T-cell lymphoma, the company is also aiming to expand the indications to other hematologic cancers (ATLL (adult T-cell leukemia/lymphoma) and AML (acute myeloid leukemia)) and solid tumors, and is currently conducting non-clinical studies in parallel.
At the 79th Annual Meeting of the Japanese Cancer Association held in October 2020, the possibility that it will become a medicine against adult T-cell leukemia lymphoma (ATL) was suggested.
The company will make efforts to increase indications in cooperation with Nippon Kayaku, which is the largest shareholder.
Future growth is expected as it is currently the only anticancer drug whose development has been completed among the pipelines of the company, which engages primarily in the development of anticancer drugs and cancer supportive care (drugs supporting cancer treatment, etc.).
(Sales Status)
◎ Japan
After being listed on the NHI drug price list (31,962 yen/bottle), the product was launched on August 22, 2022, via Nippon Kayaku, a sales partner.
◎ South Korea, Taiwan, and Hong Kong
An application for approval will be submitted after the licensing agreement is concluded.
◎ China
Negotiations for licensing are underway with several companies with the premise that the final Phase II/III clinical trials will be conducted at the licensees.
◎ U.S. and Europe
Phase II clinical trials have been completed in the U.S. In Europe, preclinical trials have been completed, and preparations are underway for Phase II/III clinical trials. Negotiations for licensing are ongoing.
◎ South America
The company possesses worldwide rights and has given exclusive rights to HB Human BioScience SAS in the Republic of Colombia, including sales in Colombia, Peru, Ecuador, Venezuela, Chile, Panama, Costa Rica and Guatemala. In Colombia, the new drug application was accepted by the authorities in December 2023. In other countries in the region, regulatory applications are being prepared.
◎ Other regions
In Europe, India, and South America are preparing for selling them under the Named Patient Program (in which pharmaceutical companies individually supply medicines to medical doctors who want to use the medicines after completing necessary procedures in a country where the medicines have not been approved and their insurance-covered prices have not been determined).
In March 2023, the company started supplying products to mainly Europe via WEP Clinical LTD.
3) “SP-03:Episil® oral liquid”
Item | Overview |
Purpose of its use | Control and relief pain of oral mucositis caused by chemotherapy or radiotherapy – Medical Device |
Characteristics/ Strength compared with competitors | *As there is no standard treatment for stomatitis caused by chemotherapy and radiotherapy, how to relieve the symptom relies on symptomatic treatment by each hospital. There is strong demand for new treatment. |
* “Episil® (SP-03)” contains no pharmaceutical agent, so there is no side effect nor interaction with anticancer agents. |
(Overview of indications)
In addition to nausea and vomiting due to anticancer agents, oral mucositis are also serious side effects caused by chemotherapy or radiotherapy.
Stomatitis can be divided into 2 types: the primary stomatitis, which is “stomatitis caused by chemotherapy directly affecting the oral mucosa” or “stomatitis resulted from local infection due to the salivary gland tissue disorder and deterioration of intraoral self-cleansing action because of impaired saliva secretion attributed to radiation exposure” and the secondary stomatitis, which is “attributed to intraoral infection due to myelosuppression resulting from a decline in the number of white blood cells.”
The incident rate of stomatitis developing during treatment using anticancer drugs is 30-40%, and that of stomatitis developing during anticancer drug treatment provided together with radiotherapy to the head and neck is nearly 100%.
Stomatitis occurs together with 300-500 inflammations arising in the course of chemotherapy or radiotherapy. The pain makes oral intake of food and water by patients difficult, which results in a decrease in physical strength. In case the symptom is severe, it will adversely affect or halt the progress of cancer treatment. Up until now, there is no established standard treatment therefore the majority of hospitals conducted palliative treatment.
(Overview of “Episil® (SP-03)”)
“ Episil® (SP-03)” is a lipid-based liquid, which is dropped and applied on the oral mucosa, which the company has been developing under the category of medical device.
(Source: Solasia Pharma)
In a few minutes after application of a proper dose to the oral mucosa, the liquid absorbs the water in the oral cavity and transforms to a bioadhesive gel which mechanically protects the affected area. The effect of mitigating the pain of stomatitis has been clinically shown to last for about 8 hours.
(Current situation of development and commercialization)
◎ Japan
Solasia Pharma submitted an application for approval in Japan in 2016 and obtained an approval of “Episil® (SP-03)” as new medical device in Japan by the Ministry of Health, Labour and Welfare on July 6, 2017. In January 2018, “Episil® (SP-03)” was approved at the 388th general meeting of the Central Social Insurance Medical Council for being covered by insurance, starting in April 2018. Following reimbursement listing, 7,660 yen per bottle(10ml) as of October in 2019, in May 2018, it was launched by Meiji Seika Pharma, which
is the licensee who holds the exclusive sales rights of “Episil® (SP-03)” in Japan.
In April 2023, Episil® (SP-03) was listed as one of the treatment methods for stomatitis in the manual for dealing with serious side effects for each disease, “Stomatitis caused by cancer treatment drugs,'' published by the Ministry of Health, Labor and Welfare.
The company had applied for regulatory approval for the addition of a new manufacturing facility (in Japan) in order to reduce manufacturing costs, and received approval as scheduled in August 2024.
◎ China
After the application for approval in May 2016, the company obtained the approval for import and sale of medical apparatus in February 2019, and started selling products in July 2019, the company dismantled the system for selling products by itself in 3 cities at the end of July 2022. Since August 1, Lee’s Pharma, which is the licensee, has engaged in sales activities throughout China. As mentioned above, additional regulatory approval has been obtained in Japan for the new manufacturing facility (in Japan), but obtaining approval for sale in China is expected to take a little longer.
Like “Sancuso® (SP-01),” the sale of “Episil® (SP-03)” has been sluggish and below expectations, but the effectiveness of the product has been highly evaluated.
In May 2021, Episil® (SP-03) was included in the Expert Guidelines on the Diagnosis and Prevention of Acute Oral Mucositis Caused by Antineoplastic Therapy newly published by Chinese Society of Clinical Oncology (CSCO), and recommended as a new treatment option.
This Guideline is regarded as having “increased the attention of clinical oncologists to oral mucositis and standardized the treatment of oral mucositis in antitumor therapy, which is of great significance,” and as Episil® (SP-03) was specifically featured, the company anticipates that this will give momentum to sales promotion in China.
Due to the product characteristics of “Episil® (SP-03),” the company will “create a market” instead of entering into the existing market.
The market is estimated to be 20 to 30 billion yen in Japan and China, and the company is aiming to acquire a 30-50% market share.
◎ South Korea
In South Korea, the company concluded a contract for introducing the exclusive right to develop and sell the medical device in South Korea with Camurus AB, which is the licensing-out company, in August 2018, applied for approval to authorities in March 2019, and acquired the approval for import and sale of medical device in South Korea in October 2019. In January 2020, the company concluded a contract for exclusive dealership with Synex Consulting Ltd. as a sales partner in South Korea. The sales started in September 2020.
◎ Other Regions
In July 2022, the worldwide business rights, including manufacturing rights, were acquired from Camurus AB.
For the time being, the company will focus on supplying products in Japan, China, and South Korea, but Solasia Pharma is also licensing out products in regions other than Japan, China, and South Korea.
【Under Development】
1) “SP-04: Intracellular superoxide scavenger PledOx®”
Item | Overview |
Indication | Chemotherapy induced peripheral neuropathy (CIPN) |
Characteristics/ Strength compared with competitive drugs | *There is currently no approved drug to prevent or treat CIPN |
*Superoxide dismutase mimetics to discompose and remove superoxide as one of reactive oxygen substance (ROS). |
While steady progress in general was being made in development of the three preceding products, the company, which had been considering in-licensing the fourth pipeline since it became listed, sought for a new drug that satisfies the following three criteri “it is aimed for the oncology,” “certain progress has been made in clinical trials,” and “the company can gain the development right both in Japan and in China.” Then, in November 2017, the company was granted the exclusive rights to development and commercialization of “PledOx®,” a drug for treating CIPN, in Japan, China, South Korea, Taiwan, Hong Kong, and Macau by Egetis Therapeutics AB (Formerly PledPharma AB, hereinafter referred to as “Egetis”) of Sweden.
(Overview of indications)
Chemotherapy-induced side effects occur not only nausea and vomiting, and oral mucositis, but also peripheral neuropathy (CIPN). CIPN is known to manifest considerable symptoms such as dysesthesia in the hands, feet, the area around lips, etc., tightness in the pharynx and larynx accompanied by difficulty in breathing and dysphagia, numbness of the limbs, hypoesthesia, and sensory ataxia, caused by major chemotherapy drugs such as platinum-based drugs and taxanes.
If these side effects appear, by suspension of administering the drugs, some of the symptoms are alleviated in 80% of the cases and completely recovered in 6 to 8 months in 40% of the case; however, as discontinuation of administration of the drugs may mean suspension of cancer chemotherapy and change in the treatment policy, treatment of CIPN is one of the crucial medical issues. There is currently no approved drug to prevent or treat CIPN.
Estimated number of patients (Japan): Approximately 70,000-180,000/year*2 (taxane preparation administration)
(Overview of “PledOx® (SP-04)”)
Egetis, the originator of “PledOx® (SP-04)” is listed on Stockholm Stock Exchange and has strengths in development of pharmaceuticals against oxidative stress-related diseases. “PledOx®” (active ingredient name:calmangafodipir) is a new active ingredient created based on “Mangafodipir,” an MRI contrast medium, which had sold in the United States and Europe.
(Development status)
The global phase III clinical trial concerning peripheral neuropathy caused by the administration of Oxaliplatin, in which Japan, South Korea, Taiwan, and Hong Kong participated alongside U.S. and European countries, began in December 2018. However, a suspension of the trial was ordered by several authorities as French National Security Agency of Medicines and Health Products (ANSM) issued a clinical hold order in addition to FDA ordering a clinical hold of the POLAR-M study conducted by Egetis in January 2020, etc.
Afterwards, Data Safety Monitoring Board performed a new safety evaluation and recommended the cessation of the registration of new study subjects and administration of the drug used in the clinical trial as multiple cases of severe allergic reactions and hypersensitivity were manifested after repeated administrations of Oxaliplatin and SP-04. As a result, Solasia Pharma and Egetis made changes to the originally planned process, implemented “data cut off” — early closing of the case data collection — in the third quarter (July-September) of 2020, following which it decided to end the global phase III clinical trial.
Moreover, as Solasia Pharma recognizes that securing the safety of study subjects is the most important regarding conducting clinical trials, it declared its policy to formulate the plan concerning PledOx® (SP-04) after performing a detailed and solid evaluation of mainly information obtained after the end of the trial regarding safety and effectiveness.
Then, on December 2020, the flash report on the global phase III clinical trial was announced.
The major evaluation items regarding efficacy were not achieved. The frequency and details of adverse effects were almost consistent with the expected ones attributable to colorectal cancer, which is the target of chemotherapy and this trial.
Since the results of this trials are limited to the data on major evaluation items, Solasia Pharma K.K. and Egetis will evaluate the details of trials results regarding secondary evaluation items, etc. and discuss the strategy for developing PledOx® (SP-04).
Based on the results of the international Phase III clinical trials (POLAR-A trial, POLAR-M trial) conducted in Japan and other countries targeting peripheral neuropathy caused by multiple chemotherapy regimens, including oxaliplatin, Solasia Pharma has decided to suspend development for this indication. To explore the possibility of developing a treatment for peripheral neuropathy caused by taxane agents, the company is conducting animal studies using a rat model of taxane 4-induced peripheral neuropathy in collaboration with Egetis (formerly PledPharma), the licensor of the drug. Although the completed animal studies did not clearly confirm the prevention of onset, the effects of SP-04 were observed in several test items, suggesting the possibility of preventing the onset of peripheral neuropathy. Non-clinical studies on taxane-induced peripheral neuropathy are ongoing.
(Licensing-out plan)
Solasia Pharma plans to give licenses in Japan and other Asian countries. In Japan, it concluded a contract for exclusive distributorship of “PledOx® (SP-04)” in Japan with Maruho Co., Ltd. (Osaka-shi, Osaka) in December 2019.
The economic conditions specified by the contract are (1) Maruho shall pay a lump-sum amount of 1 billion yen to Solasia Pharma, (2) Maruho shall pay up to 18.0 billion yen as milestone payments to Solasia Pharma according to the progress of development and sale, and (3) Solasia Pharma shall exclusively sell PledOx® (SP-04) to Maruho.
2) "SP-05:arfolitixorin"
Item | Overview |
Target Diseases | Enhancement of anti-tumor effect. Folic acid preparation (Intended efficacy: Enhancement of the anti-tumor effect of the anticancer drug fluorouracil). |
(Development Status)
Isofol, a Swedish biotechnology company and the licensor of “arfolitixorin (SP-05),” initiated an international Phase III clinical trial in December 2018 across the U.S., Canada, Europe, Australia, and Japan.
The study was aimed at comparing the efficacy of “arfolitixorin (SP-05)” in combination with 5-FU, oxaliplatin, and bevacizumab versus the standard of care (5-FU, oxaliplatin, and bevacizumab plus leucovorin) in patients with advanced colorectal cancer. However, in 2022, the final results of the trial revealed that the “arfolitixorin (SP-05)” group did not demonstrate statistically significant differences from the standard of care group for the primary and key secondary endpoints.
Following these results, Solasia Pharma temporarily removed “SP-05: arfolitixorin” from its pipeline. However, in March 2023, Isofol conducted a detailed post-hoc analysis of the AGENT trial results by external experts, as well as new preclinical studies, with the aim of resuming clinical development of “arfolitixorin (SP-05).” As a result of these comprehensive evaluations, it was concluded that “arfolitixorin (SP-05)” may demonstrate clinical efficacy under different dosage and administration than those used in the AGENT trial. In addition, Isofol announced plans to conduct a small-scale clinical trial as the first step to demonstrate the clinical efficacy of “arfolitixorin (SP-05)” with the new dosage and administration compared to the standard of care in a timely and cost-effective manner.
In response to this decision to resume clinical development on a small scale, Solasia Pharma has decided to participate in the detailed review of Isofol's clinical development program with a view to participating in future clinical trials.
In July 2024, Isofol released the results of the post-hoc analysis of the AGENT trial by external experts and a preclinical study on the dose-response relationship of “SP-05: arfolitixorin.2
* It seems that the dosage and administration of “SP-05: arfolitixorin” used in the Phase III clinical trial (AGENT trial), which did not show statistically significant results, was not optimal.
* It was found that the dosage of “SP-05: arfolitixorin” used in the Phase III trial (AGENT trial) was not commensurate with the dosage of the control group.
Based on these results, Solasia Pharma believes that:
"The Phase I b/II clinical trial, currently scheduled to start by the end of 2024, is highly likely to show positive data."
"Even with the suboptimal dosage and administration of arfolitixorin used in the Phase III trial (AGENT trial), which showed numerical differences in efficacy compared to the control group, there is a possibility of achieving even better results with optimized dosage and administration in the future."
Solasia Pharma is currently considering when to participate in Isofol's clinical development program.
(The company's policy on "SP-05: arfolitixorin")
While the development of “SP-05: arfolitixorin” seemed to have hit a roadblock, the company believes that the primary reason for the setback was the suboptimal dosage and administration. It anticipates that with an optimized dosage and administration, “SP-05: arfolitixorin” has a high potential for superior efficacy.
It is said that there are more than 150,000 patients with colorectal cancer in Japan each year, making it one of the most common cancers in Japan. Currently, most of the existing competitive products for "SP-05: arfolitixorin" are generic drugs, and the Japanese market scale is expected to be approximately 30 billion yen. New treatments have not been developed as standard treatments for colorectal cancer for over 10 years. If "SP-05: arfolitixorin" were to replace existing standard treatments for colorectal cancer, the survival rate of colorectal cancer patients would increase significantly, and the company believes that the success of the development would be highly valued.
【New candidates for products to be developed and technologies】
In addition to the five products in the pipeline, the company is working with partners on research or early-stage preclinical projects that may be positioned for development in the future.
Project | Overview |
GeneCare Project | In 2020, the company entered into an exclusive negotiation rights (option rights) agreement with GeneCare Research Institute Co. Ltd., a biotech venture company, regarding the acquisition of rights to RECQL1-siRNA, a nucleic acid drug development product owned by GeneCare Research Institute, and related technologies. The company is currently conducting joint development with GeneCare Research Institute, and depending on the progress of future non-clinical tests and new formulation development, it will consider exercising the option rights to acquire the rights. RECQL1-siRNA is a small interfering RNA (siRNA), a type of nucleic acid drug, created by GeneCare Research Institute based on licensed technology from Alnylam Pharmaceuticals, Inc. (Nasdaq: ALNY). It is believed to have a new mechanism of action that induces cell death by selectively suppressing the expression of the DNA repair helicase RECQL1, which is overexpressed in cancer cells. In multiple pharmacological tests, it has already demonstrated growth inhibition effects in various types of cancer, as well as life-prolonging effects in animal models of peritoneal dissemination that occur in advanced ovarian and gastric cancer. Solasia Pharma and GeneCare Research Institute, in collaboration with the Tei Laboratory, Graduate School of Science, the University of Tokyo, are planning further pharmacodynamic studies and new formulation development for a new siRNA sequence that is expected to have higher efficacy and safety, with the aim of transitioning to the clinical development stage. |
EditForce Project | In 2019, the company entered into a joint research and development agreement with EditForce Inc., a biotech venture company spun off from Kyushu University to secure a medium/long-term means of acquiring candidates for products to be developed. The company aims to develop new gene therapies for cancer and other diseases based on EditForce's core RNA editing technology. Currently, the company is working to establish and review various conditions for non-clinical tests to confirm the efficacy of candidate pentatricopeptide repeat (PPR) proteins created based on EditForce's RNA editing technology by selecting potential target diseases and their mutated genes. |
HikariQ Project | In 2022, HikariQ Health Inc., a biotech venture company spun off from the Tokyo Institute of Technology, entered into a capital and business alliance agreement with Solasia Pharma centered around an investment in HikariQ Health. HikariQ Health's Q-body platform technology is based on the principle that a fluorescent dye is incorporated into the interior of a Q-body antibody and quenched, but when the antibody reacts with an antigen, the incorporated fluorescent dye is ejected and emits its original fluorescence. Therefore, Q-bodies are considered to function as biosensors whose fluorescence intensity changes according to the antigen concentration. This mechanism-based immunoassay technology is expected to significantly simplify and reduce the cost of current tests using immune reactions. Additionally, the company is conducting preliminary studies on the application of this technology to next-generation antibody-drug conjugates (ADCs). HikariQ Health is also conducting joint research and development with other companies in the immunoassay business, and Solasia Pharma is collaborating with HikariQ Health to conduct preliminary studies on the development of next-generation ADCs using Q-body technology. |
Goryo Chemical Project | In 2023, the company entered into a joint development study agreement with Goryo Chemical Inc., with the aim of evaluating and considering the possibility of jointly conducting business development activities and clinical development activities related to the pharmaceutical business, including navigation drugs for cancer surgery using functional fluorescent probes based on Goryo Chemical's technology. As the first target, the company is continuing to examine the development and commercialization of a navigation drug for breast cancer (GCP-006) in Japan and the U.S. |
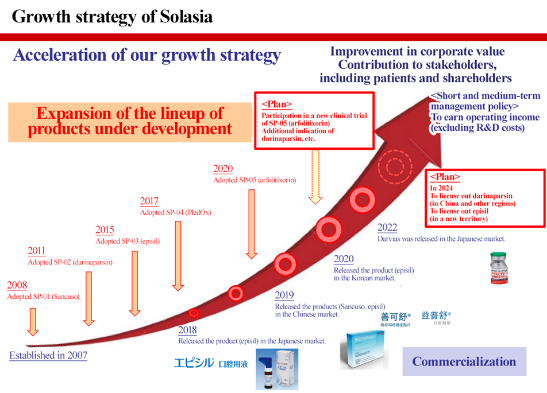
1-5 Envisioned Growth
The company will forge ahead with the sales and development of the above pipelines as planned, work toward commercialization, and achieve a positive operating profit excluding early R&D expenses. In addition, they will keep engaging in new development and continue to grow, aiming to improve the corporate value and contribute to all stakeholders including patients and shareholders.
(Source: Solasia Pharma)
1-6 “5 Characteristics” as a Biotech Company
The following 5 points characterize Solasia Pharma as a biotech company:
(1)History of establishment
Solasia Pharma started as “JapanBridge (Ireland) Limited” established jointly by ITOCHU Corporation and MPM Capital, an American VC specializing in bio business, and set up its business by licensing-in new drugs from several biotech companies and propelling development of such drugs.
At first, it mainly considered business transfer to pharmaceutical companies as its exit plan; however, taking account of the business potential and promise, the company shifted its business strategy to persistent business expansion as an independent company and took the path to public stock offering because it was essential to raise funds for research and development. Later, in March 2017, it made a public offering. As the company’s original plan was to sell the company to other companies, the pipelines it owned were comprised of prime assets that could potentially be sold to other companies for encashment even during clinical development. This means that Solasia Pharma has already established a firm business foundation since its inception.
(2) Experienced Clinical development team
Solasia Pharma does not conduct basic research or preclinical trials but in-license assets and specializes in drug creation processes carried out subsequent to the clinical development phase. The most essential thing to achieve in the process of research and development toward commercialization of pharmaceuticals is to eventually obtain approval from the authorities. This requires skills and know-how in the stage of clinical development, especially clinical trials after phase II.
Although there are a number of biotech companies in Japan, CEO Arai stands out with his deep experience and knowledge in clinical development.
The experienced clinical development team, led by CEO Arai, is a significant factor in differentiating Solasia Pharma from other companies and plays a role as a competitive edge.
(3) High rate of successful development
So far, five products including “Sancuso® (SP-01),” “DARVIAS® (SP-02)”, “Episil® (SP-03)”, “PledOx® (SP-04)”, and ”Arfolitixorin® (Sp-05)” were introduced. Three products are commercialized.
“Sancuso® (SP-01)” was released in China, “DARVIAS® (SP-02)” was released in Japan, and “Episil® (SP-03)” was released in Japan, China, and South Korea.
Such a high rate of successful development is made possible due to the following 2 points: its business model that handles only in-licensed products with a low risk of failure, and its in-house team which can handle all kinds of roles in clinical development. As mentioned above, the development staff is well aware of what are necessary for obtaining approval and therefore can conduct screening of whether or not an in-licensed product will be approved.
Their so-called “connoisseur (for screening pipelines)” has been realized by the combination of the above 2 strengths and lowers the risk of abandoning development which is the source of such a high success rate.
Analysis of the cash inflow of a new drug based on the discount cash flow (DCF) model has indicated what comprise of a majority of the total cash inflow is not contract money or milestone income, but royalties which, obviously, will be earned only after successful development of the new drug and expansion of the sales volume.
When making a proposal to Egetis (Sweden), Solasia Pharma did not necessarily have advantages over a number of its competitors in terms of prices, including contract money; nevertheless, it succeeded in in-licensing “PledOx®(SP-04).” The reason behind the success is that Egetis has thought highly of Solasia Pharma’s capabilities, including the strength of the team for producing distinct clinical trial designs, the results of development of the three preceding products, and the business performance in Asia, including Japan and China, reaching a decision that Solasia Pharma will be the best partner that will bring success in “PledOx®” in Asia.
(4) Stable business foundation
The company licensed pharmaceutical companies to sell four developed products, establishing a business portfolio in which managerial resources were concentrated on the business from the clinical development stage.
(5) Early feasibility of business
Because biotech companies in general post losses in the stage of new drug development, it is not rational to use profit and loss statements for calculating stock prices and enterprise value, and thus the DCF model is used. In case of biotech companies, however, in addition to the discount rate based on “time” which is used in the general DCF model, the success rate for each stage of clinical trials of new drugs is used as another discount rate.
In this case, the most important point is when approval can be obtained. Of the five products developed, "Episil® (SP-03)" has been launched in Japan, China, and Korea, followed by "Sancuso® (SP-01)" in China, and "DARVIAS® (SP-02)" in Japan. So, the discount rate regarding the company’s development of new drugs should be estimated lower than that of other bio-ventures.
In addition to these five points, the company has high growth potential in the Chinese market.
Understandably, large-scale pharmaceutical companies all over the world have established bases in various Asian countries including China; however, as described in its management policy, Solasia Pharma’s target of development is new products in the field of cancer and rare diseases which major pharmaceutical companies do not enter from the performance-based perspective.
These products, which have been attracting attention in the pharmaceutical market in recent years, originate from biotech ventures, but are not handled by major pharmaceutical companies, so the company, which is already highly regarded by the Chinese medical community, will be valuable in providing access to the rapidly growing Asian market for biotech ventures worldwide.
2. Second Quarter of the Fiscal Year Ending December 2024 Earnings Results
2-1 Overview of consolidated results (IFRS)
| 2Q of FY 12/23 | 2Q of FY 12/24 | YoY |
Revenue | 529 | 72 | -457 |
Gross Profit | 289 | 2 | -287 |
R&D Expenses | 192 | 223 | +31 |
SG&A Expenses | 559 | 390 | -169 |
Operating Profit | -462 | -611 | -149 |
Profit before Tax | -450 | -627 | -177 |
Quarterly Profit | -437 | -611 | -174 |
*Unit: million yen. Quarterly profit is quarterly profit attributable to owners of the parent.
The sales revenue in the second quarter of the fiscal year ending December 2024 was 72 million yen, down 457 million yen year on year.
It includes revenue from sale of “DARVIAS® (SP-02).”
The sales partners built up inventory of products manufactured at the old facility during the previous fiscal year to prevent shortages in the market during the period of shift to products manufactured at the new facility, resulting in smaller shipments of products manufactured at the new facility.
R&D expenses increased 31 million yen year on year to 223 million yen. This was mainly due to investment in the change of manufacturing facilities to reduce product cost, consideration of expansion of indications for “DARVIAS® (SP-02)” and clinical development in China, animal experiments for SP-04, and investment in candidates for products to be developed.
SG&A expenses decreased 169 million yen year on year to 390 million yen.
As a result, operating loss was 611 million yen year on year, down 149 million yen year on year.
2-2 Financial standing and cash flows
◎Main Balance Sheet
| End of Dec. 2023 | End of June 2024 | Increase/ decrease |
| End of Dec. 2023 | End of June 2024 | Increase/ decrease |
Current assets | 976 | 1,173 | +197 | Current liabilities | 293 | 303 | +10 |
Cash, etc. | 728 | 942 | +214 | Trade payables | 213 | 226 | +13 |
Trade Receivables etc. | 67 | 56 | -11 | Noncurrent Liabilities | 61 | 30 | -31 |
Inventories etc. | 122 | 138 | +16 | Total Liabilities | 354 | 333 | -21 |
Noncurrent Assets | 1,252 | 1,125 | -127 | Total Equity | 1,875 | 1,965 | +90 |
Intangible Assets | 1,117 | 1,008 | -109 | Retained Earnings | -1,336 | -1,947 | -611 |
Total Assets | 2,229 | 2,299 | +70 | Total Liabilities and Equity | 2,229 | 2,299 | +70 |
*Unit: million yen. “Cash, etc.” means cash and cash equivalents. “Trade receivables” means trade receivables and other receivables. “Trade payables” mean trade payables and other payables.
While cash and deposits increased due to the exercise of stock acquisition rights, total assets stood at 2,299 million yen, almost unchanged from the end of the previous fiscal year due to a decrease in intangible assets.
Total liabilities were 333 million yen, almost unchanged from the end of the previous fiscal year.
Total assets increased 90 million yen year on year to 1,965 million yen due to an increase in capital stock and capital surplus from the issuance of new shares and an augmentation of deficit in retained earnings.
Capital-to-asset ratio increased 1.4% from the end of the previous term to 85.5%.
3. Fiscal Year Ending December 2024 Earnings Forecasts and Future Goals
3-1 Consolidated earnings forecast
| FY 12/23 | FY 12/24 Est. |
Revenue | 617 | 1,250 ~ 1,500 |
Operating Profit | -1,139 | -800 ~ -550 |
Pretax profit | -1,135 | -800 ~ -550 |
Net Profit | -1,112 | -800 ~ -550 |
*Unit: million yen. Net profit is net profit attributable to owners of the parent.
Sales are expected to grow, decreasing loss.
◎Revenue
Sales revenue is projected to be at least 1,250 million yen, which is the sum of 500 million yen as revenues from sale of “Sancuso® (SP-01),” “Episil® (SP-03),” and “DARVIAS® (SP-02)” and 750 million yen as lump-sum revenues from the conclusion of the contract for licensing out “DARVIAS® (SP-02)” in China.
Although the impact of inventory liquidation and other factors related to the relocation of manufacturing sites for “Sancuso® (SP-01)” and “Episil® (SP-03)” will gradually weaken, it is expected to continue for the time being.
Under the assumption that the company will earn all short-term milestone income from the contract for licensing out “DARVIAS® (SP-02)” in China in 2024, an additional lump-sum payment from the contract is expected to amount to 250 million yen, so the upper limit is assumed to be 1.5 billion yen.
◎Operating expenses
For “Sancuso® (SP-01),” “Episil® (SP-03),” and “DARVIAS® (SP-02),” it is assumed that the company will incur the cost of sales through the sale of products, invest in clinical development of “DARVIAS® (SP-02)” in China, and post the amortization of intangible assets.
In addition, the company assumes operating expenses due to the investment in development of new drugs, etc. Among them, the amortization of intangible assets is projected to be 160 million yen.
As a result, the company is forecast to post an operating loss and other losses ranging from 550 million yen to 800 million yen.
3-2 Main Business Goals
◎” DARVIAS®(SP-02)”
*Securing revenues through the licensing-out in China
The company is negotiating with multiple companies for licensing-out contracts.
*Preparation for and start of clinical trials in China
They plan to start clinical trials with in the year.
*Narrowing down the target diseases of products
◎” Sancuso®(SP-01)」「Episil® (SP-03)”
*Completion of the process for relocating manufacturing sites and implementation of cost reduction measures
*Securing of revenue through the resumption of shipment volume of products
◎“PledOx®(SP-04)”
To continue animal testing to check the possibility of clinical development to include "peripheral neuropathy caused by taxane formulations” as an indication.
◎ “Arfolitixorin SP-05”
The company believes that with optimized dosage and administration, it may be able to achieve even better results. Currently, it is considering when to participate in Isofol's clinical development program.
◎Others
The company will proceed with the investment in research and development for current candidates for development and technologies, including nucleic acid medicines, gene therapy, and the creation of ADC anti-cancer drugs using novel ADC technologies.
4. Conclusions
Sales of "Sancuso® (SP-01)" and "Episil® (SP-03)" have been smaller than expected due to the transfer of manufacturing sites. Still, the high recognition of the efficacy and usefulness of these products has not changed. Although there are issues to be addressed, such as the situation in the medical field and relationships with sales partners, we will keep a close eye on the company's performance from the second half of this year onward, when the impact of the transfer of manufacturing sites will fade away.
Regarding the highly anticipated licensing-out of the Chinese rights of "DARVIAS® (SP-02)," negotiations with a potential licensee are said to be proceeding at a considerable rate. We look forward to the release of a decision on the licensee.
At the same time, we would like to focus on the progress of the clinical development program led by Isofol for "arfolitixorin SP-05" as well.
<Reference: Regarding Corporate Governance>
◎ Organization type and the composition of directors and auditors
Organization type | Company with auditors |
Directors | 5 directors, including 3 outside ones |
Auditors | 3 auditors, including 3 outside ones |
◎ Corporate Governance Report
Last update date: March 22, 2024
<Basic policy>
We believe that our mission is to contribute to the medical front including patients through our business activities as a drug development company. We also recognize that raising corporate value and returning profits to our shareholders through these business activities and fulfilling our accountability to the stakeholders are important events for achieving our mission. For these reasons, our basic policy is to effectively function corporate governance by securing “compliance” and “transparency” of management, while enhancing the monitoring and supervisory system of external directors and the audit system of corporate auditors.
<Reasons for Non-compliance with the Principles of the Corporate Governance Code>
Solasia Pharma has stated, “Our company implements all the basic principles stipulated in the Corporate Governance Code.”
This report is not intended for soliciting or promoting investment activities or offering any advice on investment or the like, but for providing information only. The information included in this report was taken from sources considered reliable by our company. Our company will not guarantee the accuracy, integrity, or appropriateness of information or opinions in this report. Our company will not assume any responsibility for expenses, damages or the like arising out of the use of this report or information obtained from this report. All kinds of rights related to this report belong to Investment Bridge Co., Ltd. The contents, etc. of this report may be revised without notice. Please make an investment decision on your own judgement. Copyright(C), All Rights Reserved by Investment Bridge Co., Ltd. |



