Bridge Report:(2183)Linical The second quarter of the fiscal year ending March 2025
 Kazuhiro Hatano CEO | Linical Co., Ltd. (2183) |
 |
Company Information
Market | TSE Standard Market |
Industry | Service |
CEO | Kazuhiro Hatano |
HQ Address | Shin-Osaka Brick Building, 6-1 Miyahara 1-chome, Yodogawa-ku, Osaka, Japan |
Year-end | End of March |
HP | https://www.linical.com/ |
Stock Information
Share Price | Number of shares issued (excluding treasury shares) | Total market cap | ROE Act. | Trading Unit | |
¥344 | 22,586,431shares | ¥7,770million | 4.3% | 100shares | |
DPS Est. | Dividend yield Est. | EPS Est. | PER Est. | BPS Act. | PBR Act. |
¥16.00 | 4.7% | ¥6.64 | 51.8x | ¥326.90 | 1.05x |
* Stock price is as of closing on December 3, 2024. Number of shares issued is the term-end figure stated in the summary of financial results for the second quarter of FY3/25 excluding treasury shares.
* ROE is based on FY3/24 results. BPS is based on the second quarter of FY3/25 results. EPS and DPS are based on the estimates of FY3/25.
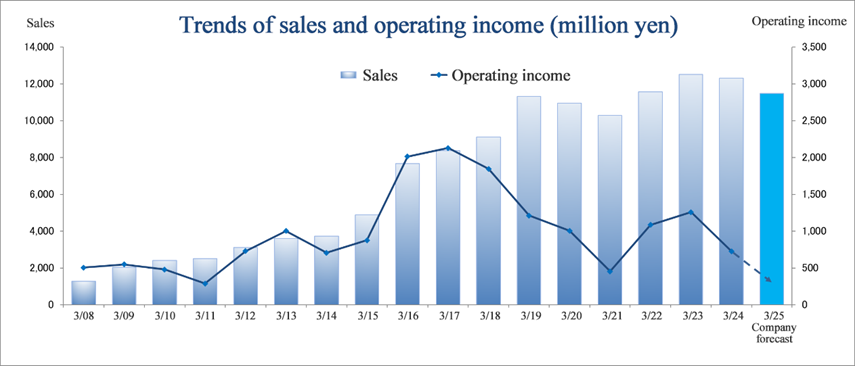
Consolidated Earnings Trend
Fiscal Year | Sales | Operating Income | Ordinary Income | Parent Net Income | EPS | DPS |
March 2021 Act. | 10,279 | 453 | 588 | 539 | 23.91 | 14.00 |
March 2022 Act. | 11,555 | 1,085 | 1,183 | 790 | 35.00 | 14.00 |
March 2023 Act. | 12,516 | 1,256 | 1,283 | 1,004 | 44.47 | 14.00 |
March 2024 Act. | 12,307 | 725 | 790 | 338 | 14.98 | 15.00 |
March 2025 Est. | 11,468 | 250 | 258 | 150 | 6.64 | 16.00 |
*Unit: Million yen.
*Estimates are those of the company.
This Bridge Report reviews on the overview of Linical Co., Ltd.’s earnings results for the second quarter of the fiscal year ending March 2025 and its forecast for the fiscal year ending March 2025.
Table of Contents
Key Points
1. Company Overview
2. Management Strategy
3. 2Q of Fiscal Year ending March 2025 Earnings Results
4. Fiscal Year ending March 2025 Earnings Forecasts
5. Conclusions
<Reference:Regarding Corporate Governance>
Key Points
- In the first half of the fiscal year ending March 2025, sales decreased 10.5% year on year, and they posted an operating loss of 192 million yen (an operating income of 421 million yen in the same period of the previous year). Overall sales declined, as the sales in the U.S. grew significantly, but the sales in other regions dropped. In terms of profit, the profit in the U.S. increased significantly, but the operating income in other regions was negative, so they posted an overall operating loss.
- The company downwardly revised the earnings forecast for the fiscal year ending March 2025 on November 14. The latest forecast of the company calls for a 6.8% y/y decrease in sales and a 65.6% y/y drop in operating income. In terms of sales, the sales in the U.S. exceeded the forecast thanks to the steady progress of existing projects, but they are struggling to receive new orders in Europe and Japan as assumed, and some development projects in Japan have been cancelled. In addition, sales were affected by the delay in progress of ongoing projects and business negotiations for new deals caused by the large-scale strike by medical doctors that started in South Korea around February of this year, so sales are projected to fall below the initial forecast. In terms of profit, they are controlling personnel expenses by adjusting staffing and optimizing SGA at all business establishments, but profit is forecast to be affected significantly by the declines in sales in Japan and other Asian countries. On the other hand, there is no change to their plan to pay an ordinary dividend of 16 yen/share, up 1 yen/share from the previous fiscal year.
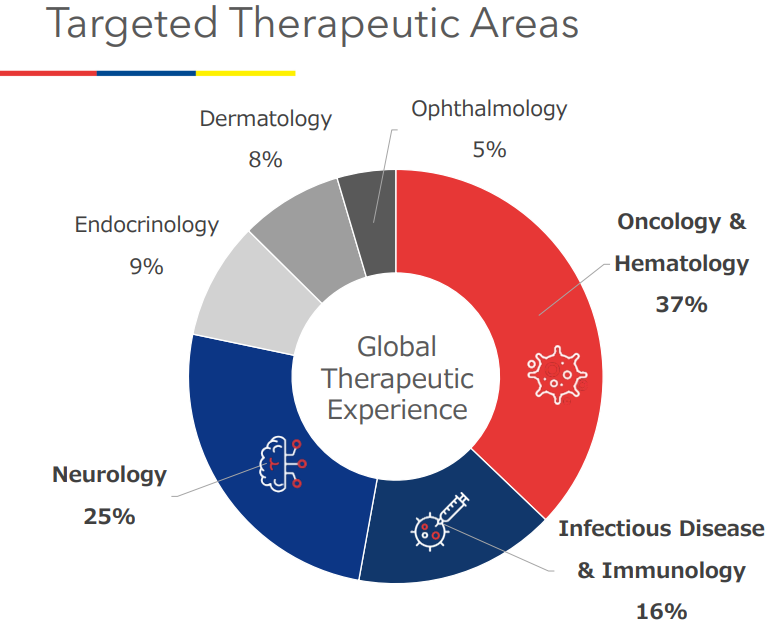
- The company has been concentrating on the fields of oncology, the central nervous system (CNS), and immunology, in which they possess strong competitive advantages, since the establishment of the company. In addition to these three fields, they plan to expand their business in the ophthalmic and dermatological fields, where needs will grow in the aged society, and the fields of regenerative medicine and digital medical apparatus, which are new modalities. It is noteworthy whether they can produce expected results in such promising fields.
1. Company Overview
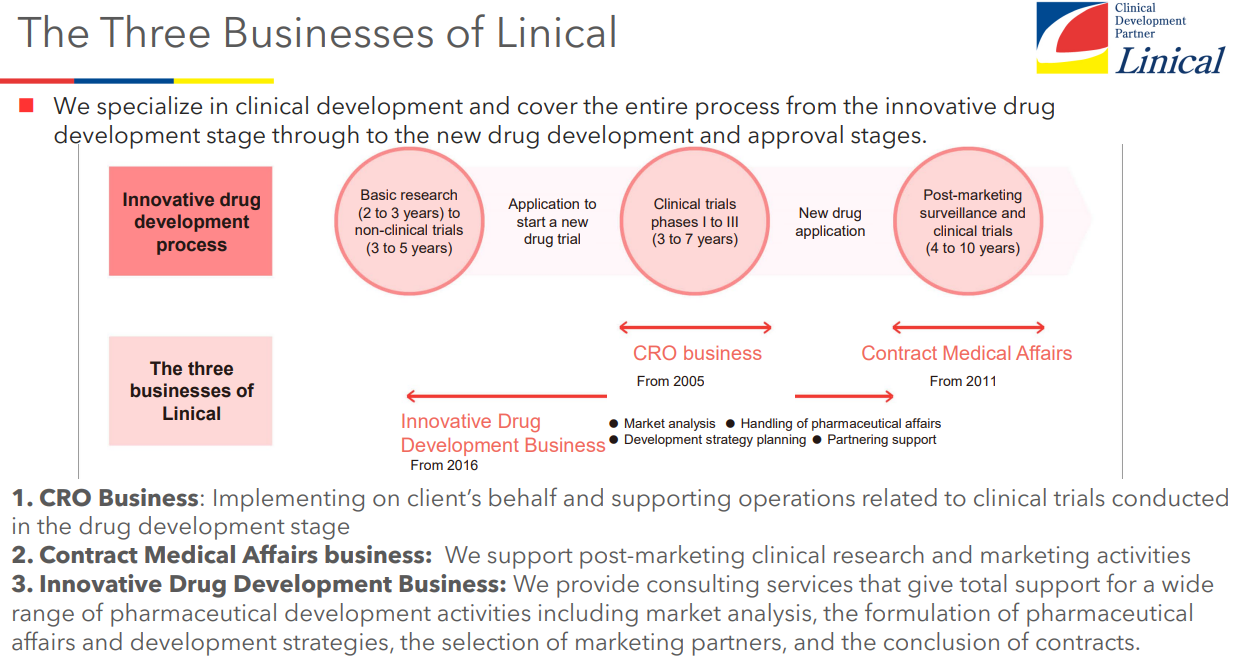
The company is a global contract research organization (CRO) based in Japan, which provides a comprehensive range of services ranging from the initial phase of clinical trials to post-marketing studies as a professional in development of pharmaceutical products. Linical Co., Ltd. provides contract research organization (CRO) services that support the drug development processes of pharmaceutical companies on an outsourced consignment basis, and sales and marketing functions for pharmaceutical products and post market launch clinical research and surveys on a consigned basis in the Contract Medical Affairs Business (CMA).
Pharmaceutical products are subject to approval of the Ministry of Health and Welfare prior to their sales, and efficacy and safety of pharmaceutical products must be confirmed through clinical trials prior to their approval. Companies providing clinical trial support services are known as contract research organization (CRO) service providers. In addition, there is a need to conduct surveys and clinical research after pharmaceutical products have been launched into the market and contract medical affairs is a service provided to support these efforts.
Linical has conducted various efforts to eradicate oncology, central nervous system and other diseases globally since its founding, and it has deployed its CRO Business in therapeutic areas where there is strong demand for new drug development. These are highly difficult areas, and Linical is able to support high level clinical trials in these areas by the company’s knowledgeable and experienced experts. In addition, Linical focuses its efforts up the new drug development support and Contract Medical Affairs Business, approval application support and post approval marketing and clinical research, and post market survey support services, which exceed the traditional definition of outsourcing and is now considered to be part of a wider range of consulting services provided to customers as a "true clinical development partner". Furthermore, amidst the advance of globalization and large-scale pharmaceutical product development, the Linical Group can provide "one stop shopping" type comprehensive services for large scale global products. Consequently, Linical is able to play the role of a strategic business partner by providing total support to help raise the competitive advantage of customers in the market and to help pharmaceutical companies develop new future business opportunities.
【Management philosophy】
The management philosophy is “Linical promotes the greater wellbeing of all our stakeholders—patients, clients and employees— we strive constantly to offer professional, high-quality services to support all aspects of new drug development.”

The blue color represents “Integrity&Honesty,”
the red color “Unending Enthusiasm,” and
the yellow color “Continuing Spirit of Inquiry.”
This corporate logo depicts the company’s hope of pursuing the wellbeing of patients around the world through business, and the company has the mission to “wings to new drugs.”
【Corporate History】
Linical Co., Ltd. was established in June 2005 by nine members who worked at Fujisawa Pharmaceutical Co., Ltd. (Currently known as Astellas Pharma Inc.) on the development of immunosuppressant drugs. Established with the objective of becoming the ideal drug development outsourcing (CRO) company from Osaka, Linical focused its efforts upon the realms of central nervous system diseases (CNS) and oncology since its founding, and received one of its first orders from Otsuka Pharmaceutical Company shortly after its establishment. Thereafter, the Company fortified its staffing as part of its efforts to strengthen its order taking capabilities. In addition, Linical is benefitting from the bountiful experiences of its employees in the realm of oncology pharmaceutical product development and experiences having worked at foreign pharmaceutical companies. Consequently, Linical is successfully expanding orders in the near term.
With its advance into the site management organization (SMO, clinical trial facility support organization) business, Aurora Ltd. was turned into a subsidiary in January 2006. However, all shares held in Aurora were later sold in May 2007 in order to focus management resources upon the CRO Business. In July 2008, Linical USA, Inc. was established in California, United States to provide support to Japanese pharmaceutical companies seeking to enter the United States market. Also, in October of the same year, Linical listed its shares on the Mothers Market of the Tokyo Stock Exchange, and subsequently moved its listing to the First Section of the Tokyo Stock Exchange in March 2013. In May 2013, Linical Taiwan Co., Ltd. and Linical Korea Co., Ltd. were established in Taiwan and Korea respectively. In April 2014, Linical teamed up with its Linical Korea to acquire the Korean CRO company P-pro. Korea Co., Ltd. On October 29, 2014, all of the shares of Nuvisan CDD Holding GmbH, which conducts CRO Business in Europe, were acquired and it was converted to a 100% owned subsidiary effective on December 1, 2014. In order to strengthen the collaboration within the Group, the company name of Nuvisan CDD was changed to Linical Europe GmbH. In addition, Linical U.K. Ltd. was established in March 2016, and a local subsidiary called Linical Poland SP. Z.O.O. was also established in October of the same year. Moreover, LINICAL Czech Republic s.r.o was established in September 2017. In addition, Accelovance, Inc. was acquired in April 2018 and its company name was changed to Linical Accelovance America, Inc. In addition, Linical Hungary Kft. was established in March 2019, and Linical China Co., Ltd. was established in May 2019. Furthermore, the company further strengthened their system for undertaking global joint clinical trials, through the enhancement of their business in the European region by integrating the European subsidiary of Linical Accelovance America, Inc. (LAA) into LINICAL Europe GmbH in December 2019, and the establishment of a Shanghai branch in February 2020. In April 2020, Linical Benelux BV and Linical Accelovance Europe BV were merged to form Linical Netherlands BV, and Linical China Co., Ltd. and Linical Accelovance China Ltd. are scheduled to be integrated in the fiscal year ending March 2023. The company grew steadily through overseas mergers and acquisitions, and achieved record sales consecutively in fiscal year ended March 2022 and fiscal year ended March 2023. In the fiscal year ended March 2024, profit declined due to the decrease in sales in Japan and Europe, and in the fiscal year ending March 2025, too, sales and profit are projected to decline due to the drop in sales in Japan and other Asian countries.
*Produced by Investment Bridge Co., Ltd. with reference to disclosed material.
【Strengths】
Global one-stop full services |
They have established an international development system, to offer services in Europe, the U.S., and Asia, mainly Japan. They offer comprehensive services, including the planning of development of pharmaceutical products, monitoring, regulatory affairs, and data management, in a one-stop manner. |
To create, develop, and improve medicines thoroughly |
They deal with all processes, including the development of new medicines and lifecycle management after approval, as professionals in development of pharmaceutical products |
A track record of conducting highly difficult tests |
They concentrate on the fields of oncology, neurology, immunology, etc. in which unmet medical needs are significant and clinical trials are very difficult, and have plentiful experiences. Currently, they are expanding their business in the fields of regenerative medicine, ophthalmology, dermatology, etc. |
【Business Description】
As a global CRO founded in Japan, the company operates primarily in Japan, and also in Asia, Europe and the U.S, providing a comprehensive range of services ranging from the drug discovery stage to clinical development to post-marketing drug development. The company has extensive experience and achievements in the trending areas of drug development, notably in oncology, neurology , and immunology.
A CRO is an organization that receives requests from pharmaceutical companies and others to act on their behalf and provide support for clinical trials conducted during the development phase of a pharmaceutical product. It is an organization with high expertise in clinical trials and is a professional in the field of drug development. The scope of work includes monitoring activities to ensure that clinical trials are conducted in compliance with regulatory requirements and the clinical trial protocol, including data management and medical writing activities.
Linical mainly conducts contract research organization (CRO) business, post market launches clinical trial and clinical research and marketing support activities in the Contract Medical Affairs Business, and new drug development support business. As a true partner, the company contributes to the maximization of the value of the medical drugs by helping the procedure from the non-clinical tests to clinical development and after-release surveys and clinical trial, and making it possible to shorten the time needed to start selling the drugs and prolong the life-cycle of the products. On top of that, the company supports not only pharmaceutical companies but also the bio-ventures in various ways including exit strategies.
(Source: Linical)
CRO Business (Contract Research Organization)
The CRO business undertakes part of the clinical trial operations conducted by pharmaceutical companies, including monitoring, data management, medical writing, pharmacovigilance, biostatistics, and quality control. The company employs highly skilled and experienced staff with the aim of supporting high-quality, highly efficient clinical trials that will lead to the rapid launch of new drugs on the market. The company has opened facilities in Asia (Korea, Taiwan, Singapore, China), Europe and the United States to be able to respond to growing demand for global studies. They offer one-stop services, including the design of clinical development plans, monitoring, data management, biostatistics, pharmacovigilance, and support for application for approvals for pharmaceutical products and medical apparatus. Among the new drug development projects spanning from 10 to 20 years, Linical is focused on the processes of “Phase II” and “Phase III” that require 3 to 7 years targeting patients who are particularly important in clinical trials, and it provides “monitoring” services that are the core of the clinical trials in the contract-based business style in conjunction with “quality control” and “consulting.” It collects highly reliable data and supports the rapid and reliable development of new drugs.
In addition, the company offers high-quality services in the fields of schedule management, standard procedure documents for clinical trials, compliance with GCP, the reliability of data and case reports, etc.
* Global jointly conducted clinical trials
“Global jointly conducted clinical trials” refers to conducting clinical trials simultaneously in multiple countries or regions in order to develop new drugs on a global scale and aim for early launch.
*GCP (Good Clinical Practice)
“GCP” is the international rule the companies are supposed to obey when they conduct the clinical trial. It is enacted by Ministry of Health, Labor and Welfare as a ministerial ordinance so that they can conduct it properly in Japan.
Since the establishment of the company, they have been dealing with very difficult clinical trials in fields where there are significant unmet medical needs and many patients are waiting for the creation of new medicines, and concentrating on the fields of cancer, immunity, and the central nervous system.
(Source: Linical)
Contract Medical Affairs Business
In the contract medical affairs business, the company provides support for the organizational structure and construction of corporate and doctors-led clinical research, as well as planning for surveys, monitoring, and auditing services for post-release clinical trials and investigations. The Clinical Trials Act is enacted, and the environment surrounding clinical research is changing drastically. Under this circumstance, to obtain information in a timely manner and be the best partner for the medical affairs department of pharmaceutical companies, Linical provides full-service support including data management and biostatistics with a focus on monitoring and research administration works of clinical trials. It has a policy to respond to the latest regulations and contribute to the creation of evidence in the challenging areas based on the know-how cultivated in the past development works.
Innovative Drug Development Business
Following the existing CRO Business and Contract Medical Affairs Business, Linical is cultivating the third business called Innovative Drug Development Business. In the innovative drug development business, the company provides consulting services to support entry into the Japanese market. This business is mainly operated by employees who are involved with licensing, business development, clinical trials development, development pharmacy, and marketing at major domestic pharmaceutical companies and have abundant results and experiences in determining developed products, introduction and derivation negotiation, and clinical development. In the Innovative Drug Development Business, 3 types of consulting services: a) market analysis of developed products, b) support for PMDA consultations, and c) partnering and licensing support, are provided. With these experiences as a weapon, they support pharmaceutical companies and bio technology companies inside and outside Japan in the broad therapeutic field.
【Services】
Strategy for drug development
Protocol Development and Study Design | The company has a track record in protocol development and study design, resulting in numerous successful clinical developments. It formulates a plan according to the project's needs and develop a roadmap for high-quality, efficient testing while mitigating risks. |
Regulatory Consulting | The company is an expert in global drug development and provides world-class consulting services in pharmaceutical affairs. It proposes the most appropriate strategies and assists in the most cost-effective and fastest response in the pharmaceutical process. |
Regulatory Affairs | The pharmaceutical affairs team at the company has extensive expertise and experience in supporting early to late-phase clinical development. Furthermore, they understand both regulatory and clinical affairs and provide comprehensive support for drug and medical device development, including regulatory application strategies, support for meetings with regulatory authorities, as well as coordination during the initiation of clinical trials. The company has extensive experience working with clients in the U.S, Europe, and Asia. |
Quality Assurance | The company places the highest emphasis on quality. It offers services worldwide, from developing SOP to QA consulting to auditing. |
Medical Writing | Medical writing is essential for clear communication and consistency in the preparation of documents related to clinical trials, as well as to ensure the safety of subjects and deal with regulatory reviews. The company aims to provide additional value through high-quality medical writing by utilizing its high level of expertise to meet the client's requirements. |
Clinical trials
Feasibility and Study Set-up | To conduct feasibility studies and select medical facilities to initiate clinical trials more swiftly. With a practical and strategic approach based on extensive field experience, the company works closely with clients to understand their objectives, propose innovative solutions, and initiate clinical trials to ensure prompt completion of incorporation. |
Project Management | The experienced project team of the company works as a partner with clients, assisting them until the completion to ensure that the trials are on schedule, within a budget, and obtain data of the expected quality. Furthermore, the company accompanies clients on projects to ensure that their needs are satisfied, responding quickly to their requests while leveraging its previous experience. |
Pharmacovigilance | The pharmacovigilance of the company is a global team of experts. They provide rapid and accurate support to clients in responding to safety information. |
Clinical Monitoring | Monitoring is essential for protecting the human rights and safety of subjects as well as ensuring regulatory compliance, data quality, and the integrity of clinical trial results. Since its establishment, the company has specifically dedicated itself to monitoring and is renowned for its quality among its clients. |
Data Management and Biostatistics | The data management and biostatistics of the company are aimed at providing both deep insights and efficiency at every stage of the process. The professionals of the company perform everything ranging from consulting to full-service data management to statistical design. |
Patient Recruitment | Finding suitable subjects for clinical trials is not easy, and the inclusion of cases is the major factor in the success or failure of a clinical trial, which can lead to significant delays in the trials and losses. It is essential for the success of a clinical trial that the recruitment plan for subjects is carefully considered. |
Training | The CRA training offered by the company provides more practical training, with lectures given by skilled professionals and clinical trial managers (CTMs) from clinical sites, enabling trainees to become excellent clinical development monitors with exceptional monitoring skills. |
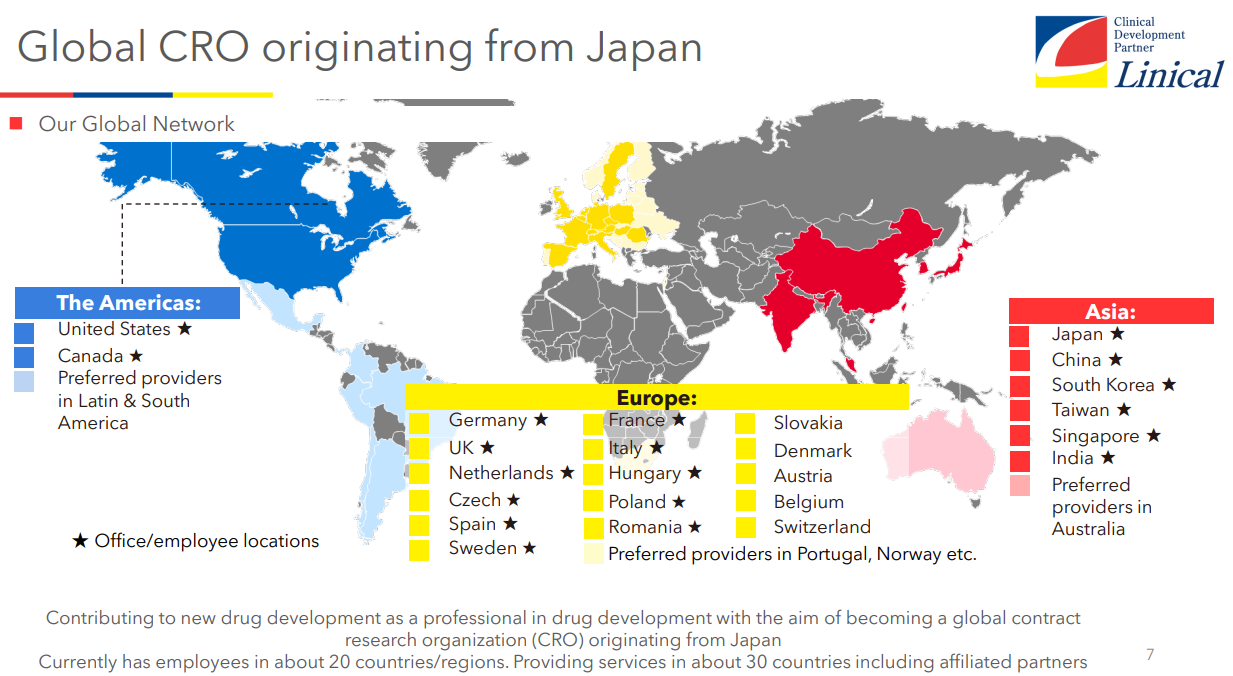
【Global expansion】
The company is a global CRO based in Japan, with a focus on Japan, it is operating its offices around the world, including Asia, Europe and the U.S. The company operates in about 20 countries/regions, and about 30 countries/regions if countries where it can provide services through partners are included. Experts in each function, who are familiar with local regulations and customs, work together globally to provide detailed services customized to each and every project. On July 1, 2024, they established a new office in New Jersey, the U.S., and on July 9, 2024, they established Linical Australia as a 100% subsidiary serving as the first base of the company in the Southern Hemisphere.
LINICAL Global Base “Three Main Operating Regions of “Japan and Asia, United States, Europe”
(Source: Linical)
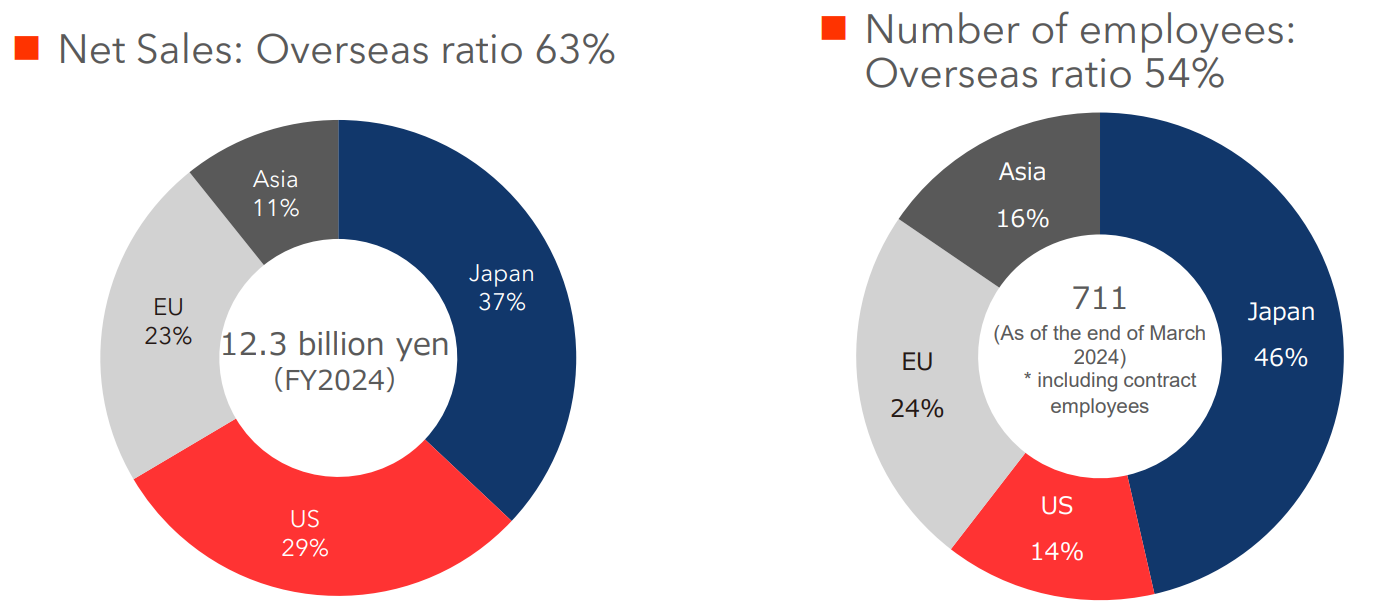
(Source: Linical)
In the fiscal year ended March 2024, the overseas ratio was 63% for sales and 54% for the number of employees.
【Order backlog by region】
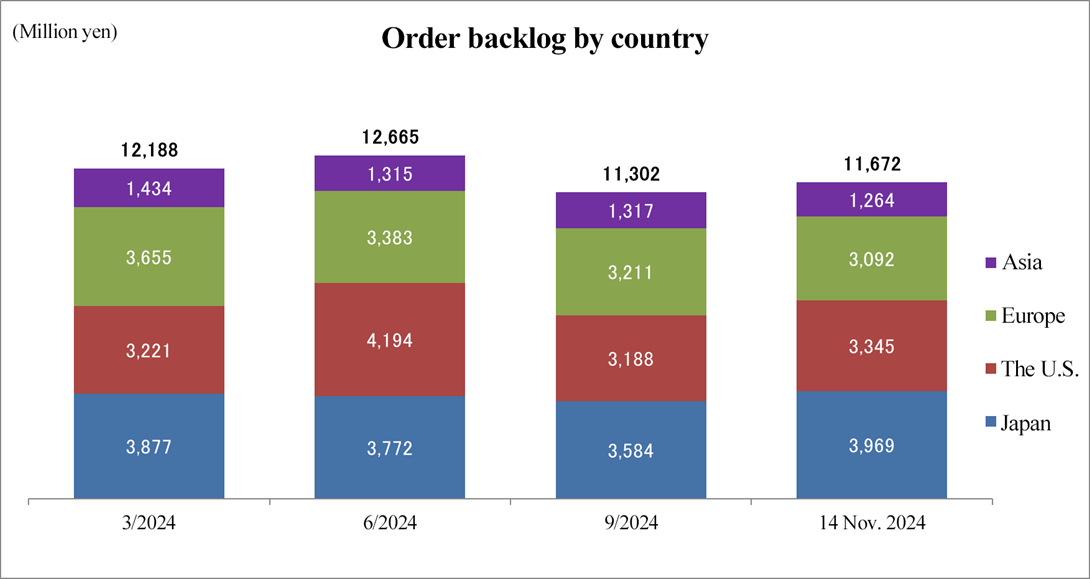
*Produced by Investment Bridge Co., Ltd. with reference to disclosed material.
Order backlog refers to the balance of the amount of orders received for undertaking of tasks for which contracts have been already concluded. It shows sales that will be earned in the next one to five years and is an indicator that serves as the basis for the corporate group's future earnings forecasts. As of November 14, 2024, order backlog stood at 11.6 billion yen, down 4.2% from the end of the fiscal year ending March 2024. However, it is now on an upward trend from the fiscal year ended September 2024 due to some informal contracts that have not yet been signed. Two major orders are to be placed in Japan, and they still receive many inquiries from biotech businesses in the U.S. Additionally, the integration of management in Europe and the U.S. is demonstrating synergies in Europe, resulting in the undertaking of new projects.
<Order backlog in each region>
Japan and Asian countries | ◆jAs of November 14, the order backlog in Japan exceeded that at the end of the fiscal year ended March 2024, despite continued harsh market conditions. ◆In South Korea, they are struggling to receive new orders due to the strike by medical doctors, and the impact on the company will augment if it is prolonged. ◆In cooperation with subsidiaries in Europe and the U.S., the company will persistently continue sales activities such as proposing that overseas biotech businesses should enter the Japanese and Asian markets. |
The U.S. | ◆Due to the undertaking of new projects and contract revisions for additional man-hours in ongoing projects, order backlog grew from the end of the fiscal year ended March 2024. ◆There are many inquiries from biotech businesses, and the company will continue to strengthen sales to secure many orders. |
Europe | ◆The integration of management in Europe and the U.S. is demonstrating synergies, resulting in the undertaking of new projects. ◆To strengthen sales personnel and receive more orders in Europe. |
2. Management Strategy
According to "IQVIA The Global Use of Medicines 2024 OUTLOOK TO 2028," the global pharmaceutical market is expected to grow at an average annual rate of 6-9% until 2028. Furthermore, their share in the global market in 2028 is expected to be 45.1% in the U.S., 13.2% in Europe (only Germany, France, Italy, the UK, and Spain), 8.8% in China, and 3.3% in Japan. The company believes that it is essential to expand its business in the U.S., the largest market.
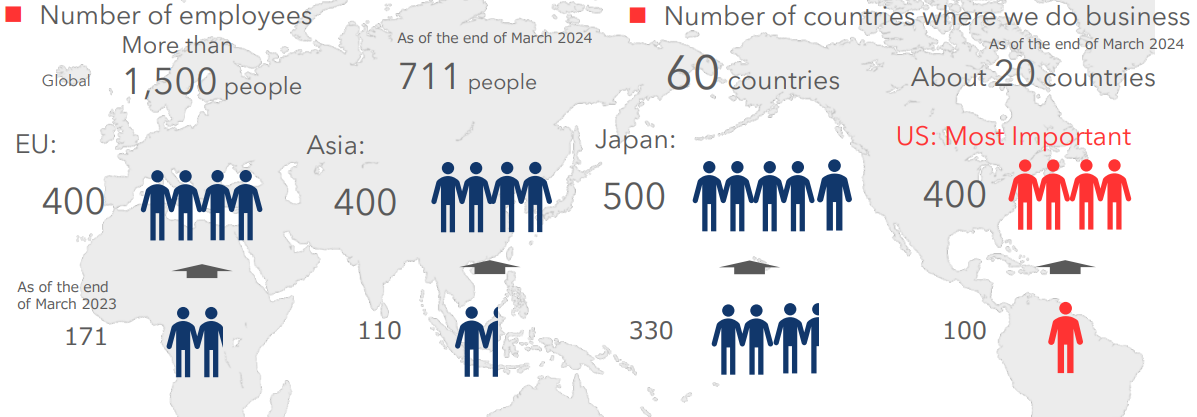
【Medium and Long term Goal】
(1) To build a system with more than 1,500 employees where there will be 500 in Japan, 400 in Asia, 400 in Europe, and 400 in the U.S.
(2) To maintain and improve profitability while making growth investments (including M&A) in all main business regions
(3) The company will expand its business to about 60 countries around the world.
(Source: Linical)
【Initiatives to strengthen profitability】
The company is promoting the following actions as a top priority in order to achieve sustainable sales growth and increase profit margin: (I) expanding the overseas business further; (ii) growing the clientele; (iii) expanding the therapeutic areas; and (iv) expanding the service areas. Additionally, the company's objective is to keep endeavoring to use new treatments, including regenerative medicine and applications, in view of changes in the market environment.
①Expanding the overseas business further
The company will expand its global service network, focusing on the U.S., the most important market.
The U.S. |
◆To increase employees and accelerate the discussions for speedy completion of M&A. |
Europe |
◆To promote management integration with the companies in the U.S. and strengthen the systems in major European countries (Germany, Italy, France, the UK, and Spain). ◆Established a development system in the Scandinavian Peninsula (Sweden), where the company had not yet entered. |
Asia-Pacific Region |
◆To invite European and American biotech companies to participate in FIH (First in Human) testing, primarily in the recently created Australian base, in order to pave the way for the undertaking of international collaborative trials from the next phase onward. *The Australian clinical trial environment is distinguished by three factors: (1) a very short timeframe from the application for a clinical trial to its start; (2) the existence of several world-class Phase I clinical trial centers; and (3) a preferential tax system for pharmaceutical and biotech companies conducting clinical trials in the nation. As a result, many western biotech companies conduct Phase I in Australia before moving on to Phase II and III in Europe and the U.S. |
②Growing the clientele
To expand the focus from major Japanese pharmaceutical companies to overseas pharmaceutical and biotech companies.
Emerging biopharmaceutical companies in Europe and the U.S.: Leading players in drug discovery and priority targets |
◆To expand trade as business grows in Europe and the U.S. Some of the repeat customers are companies that have grown significantly from ventures. ◆They are inviting more companies to the Japanese market and strengthening the cooperation and activities between the innovative drug development business of the Japanese head office and the European and U.S. sales teams. *The innovative drug development business also plays a role of supporting the entry of overseas customers into Japan, with 47% of customers from overseas. The transactions with overseas customers are increasing in a wide range of therapeutic areas and product types. |
Major pharmaceutical companies in Europe and other countries |
◆To strengthen the structure of the sales team in Europe and the U.S. to acquire early-stage and real-world trials |
Medium-sized Japanese pharmaceutical companies |
◆To strengthen sales activities in response to the growing interest in the Chinese market. |
③Expanding the therapeutic areas
In addition to the three focus areas, the company will aim to expand into the fields of ophthalmology and dermatology, where needs are growing in the aging society, and the fields of new modalities, such as regenerative medicine and digital medical devices.
To focus on oncology, central nervous system (CNS), and immunology since establishment |
◆In the oncology area, commissioned work is expanding at overseas subsidiaries. In the CNS area, the company has a strong presence in Japan and Asia. ◆In the autoimmune and rare diseases area, the company receive orders stably. |
To strengthen the domains of ophthalmology and dermatology with growing needs in the aging society |
◆To undertake more projects in the fields of ophthalmology and dermatology in Japan and other Asian countries |
Approaches to new drug discovery modalities |
◆The number of inquiries on regenerative medicine, therapeutic applications, and digital medical devices is increasing, and in addition to the innovative drug development business, the number of clinical trial contracts is steadily increasing. *The company is providing comprehensive support for the domestic Phase I/II clinical trials of Heartseed Inc., a bio venture founded by Keiichi Fukuda, a professor of Keio University. The company has established "myocardial regenerative medicine," a treatment method in which microtissues (myocardial cells) derived from allogeneic iPS cells are transplanted into the heart, and is developing HS-001 to contribute to patients with severe heart failure. |
④Expanding the service areas
To strengthen functions to provide full services for the drug discovery stage and all phases of clinical trials.
Innovative drug development business |
◆Consultation projects for the transition from non-clinical to clinical trials are increasing, and the company is planning to increase the number of employees. |
Data management/ biostatistics |
◆To strengthen recruitment and training of data management and biostatistics specialists who are important in the planning of clinical trials in the U.S., Japan, and South Korea |
To expand the scope of collaboration for functions not available in the company |
◆Apart from establishing a network of system-related partners required for distributed clinical trials (DCT), etc., the company will encourage cooperation with external experts to enhance consulting services for pharmaceutical affairs (consultation with regulatory bodies, submission of clinical trial notifications, etc.) and development CMC (physical properties, manufacturing processes, and quality stability of investigational drugs) of regenerative medicinal products, etc. |
3. 2Q of Fiscal Year ending March 2025 Earnings Results
(1) Consolidated results
| FY3/24 1H | Ratio to sales | FY3/25 1H | Ratio to sales | YoY | ||||
Sales | 6,064 | 100.0% | 5,426 | 100.0% | -10.5% | ||||
Gross profit | 1,984 | 32.7% | 1,314 | 24.2% | -33.8% | ||||
SG&A | 1,563 | 25.8% | 1,507 | 27.8% | -3.6% | ||||
Operating Income | 421 | 6.9% | -192 | -3.6% | - | ||||
Ordinary Income | 483 | 8.0% | -239 | -4.4% | - | ||||
Parent Net Income | 178 | 3.0% | -280 | -5.2% | - | ||||
*Unit: million yen
*The figures include figures calculated by Investment Bridge Co., Ltd., and may differ from actual figures. (Abbreviated hereafter)
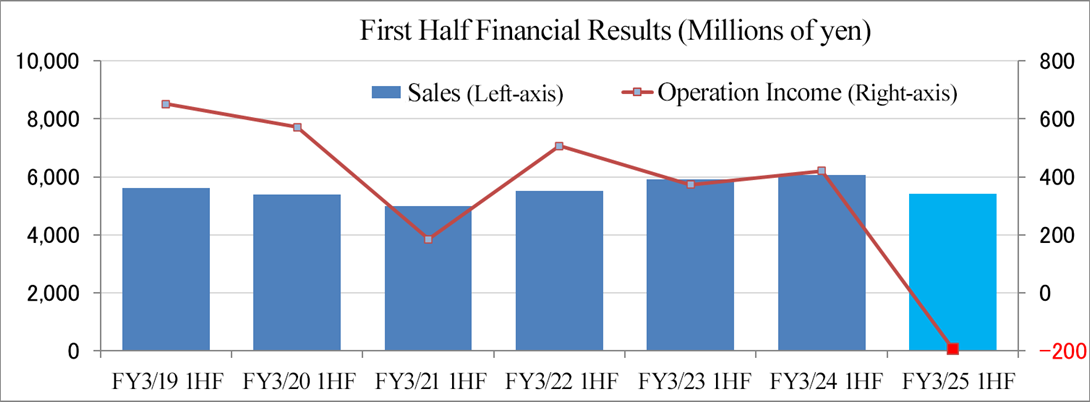
Sales decreased 10.5% year on year, and an operating loss of 192 million yen was posted.
Sales decreased 10.5% year on year to 5,426 million yen, and they posted an operating loss of 192 million yen (an operating income of 421 million yen in the same period of the previous year). Overall sales declined, as the sales in the U.S. grew significantly, but the sales in Japan and other Asian countries dropped.
In terms of profit, the operating income in the U.S. increased significantly due to the healthy sales, and the operating loss in Europe shrank from the same period of the previous fiscal year, but an operating loss was posted in Japan and other Asian countries due to the significant drop in sales, so the company posted an overall operating loss. Gross profit margin declined 8.5 points year on year to 24.2%. SGA decreased 3.6% year on year, as they optimized SGA in all business establishments. As non-operating expenses, they posted an exchange loss of 64 million yen (an exchange gain of 69 million yen in the same period of the previous year), so ordinary loss amounted to 239 million yen, exceeding the operating loss. There was no extraordinary gain or loss.
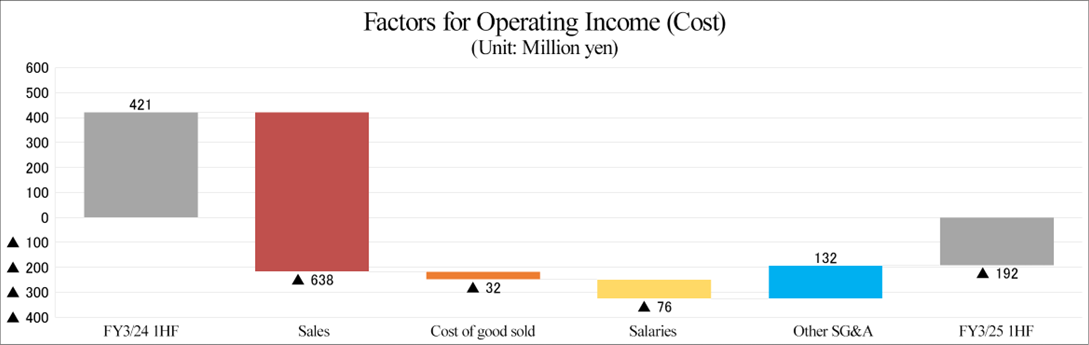
*Salaries include salaries & allowances, provision for bonuses, costs for retirement benefits, etc.
*Produced by Investment Bridge Co., Ltd. with reference to disclosed material.
Sales and profit by segment
In the CRO Business, sales decreased 9.3% year on year to 5,155 million yen and operating income decreased 39.8% year on year to 831 million yen.
In the Contract Medical Affairs Business, sales decreased 29.0% year on year to 270 million yen, and operating loss was 24 million yen (operating income of 109 million yen in the same period of the previous year).
(2) Performance trend in each region
| FY3/24 1H | FY3/25 1H | ||||
Sales | Operating income | Sales | YoY | Operating income | YoY | |
Japan | 2,682 | 370 | 1,892 | -29.5% | -215 | - |
U.S. | 1,998 | 304 | 2,513 | +25.8% | 410 | +34.9% |
Europe | 1,578 | -118 | 1,565 | -0.8% | -54 | - |
Korea | 442 | 14 | 375 | -15.1% | -73 | - |
Taiwan | 50 | -16 | 42 | -15.8% | -28 | - |
China | 165 | 1 | 115 | -30.5% | -7 | - |
Adjustment | -853 | -134 | -1,077 | - | -223 | - |
Total | 6,064 | 421 | 5,426 | -10.5% | -192 | - |
*Unit: million yen
*Amortization of goodwill is recorded as an adjustment. “Sales” means the value before exclusion of internal transactions.
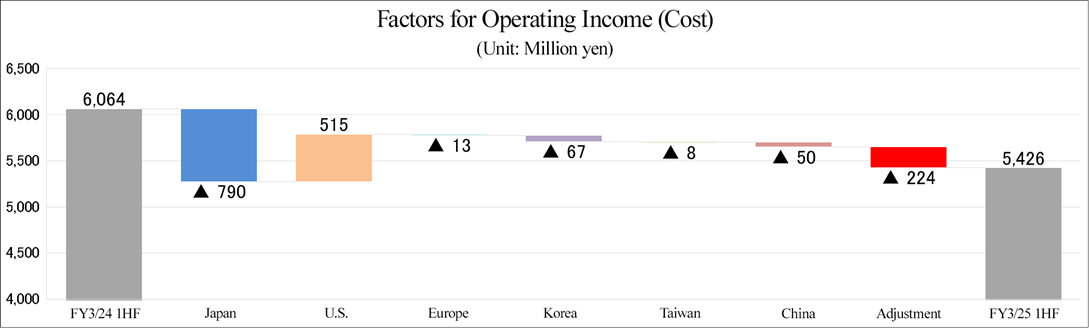
*Produced by Investment Bridge Co., Ltd. with reference to disclosed material.
【Japan】
Sales decreased year on year and an operating loss was posted in Japan. The failure to receive orders as planed and the impact of contract revisions to shorten the timeframe in the previous fiscal year, as well as the cancellation of several ongoing projects, resulted in a significant y/y drop in sales. Additionally, because of the drop in sales, the company reported an operating loss. In the Japanese pharmaceutical industry, the company is persistently continuing sales activities by inviting overseas biotech businesses to enter the Japanese market in cooperation with the businesses in Europe, the U.S., and Asia, despite the fact that the market environment stands severe due to structural reforms like a series of early retirement offers by every company. By taking steps to boost employee utilization rates and carefully examining SG&A, the company is further attempting to enhance its performance.
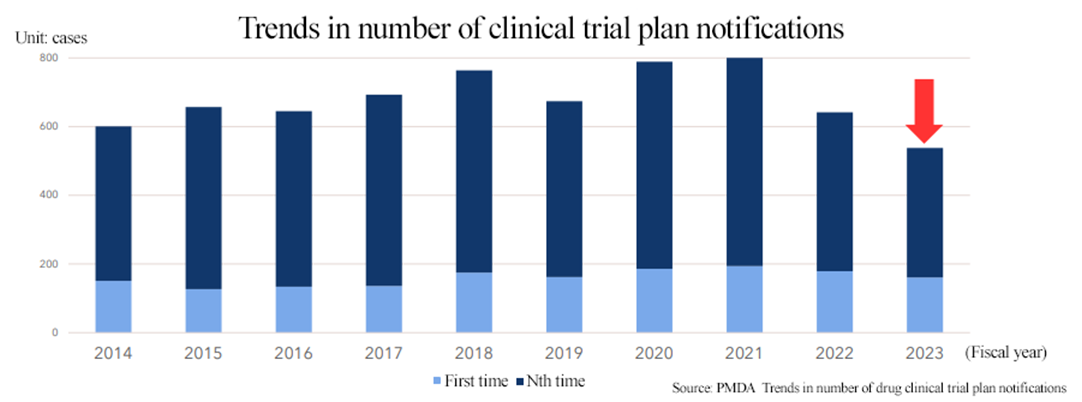
(Source: Linical)
In FY 2023, the number of clinical trial plan notifications fell to the lowest level in 10 years, and the market environment in Japan continues to be severe.
【Korea】
In South Korea, sales fell year on year, and an operating loss was recorded due to a decrease in sales as a result of revisions in contracts for ongoing projects, as well as delays in the progress of several projects. The situation remains severe, having a major influence on clinical trials as a result of the strike by medical doctors in South Korea. Since these developments are affecting both the completion of ongoing projects and orders for new ones, the company is keeping a careful eye on them.
【China】
In China, delays in the commencement of new projects and a drop in revenues as a result of the completion of existing projects led to a y/y decline in sales, and an operational loss was reported.
【Taiwan】
In Taiwan, the company struggled to undertake new projects and was unable to make up for the impact of the cancellation of existing projects and the completion of projects acquired in the previous year. As a result, sales dropped and operating loss increased year on year. However, there are signs of improvement in sales, such as the receipt of informal agreement for international trials from Taiwanese biotech businesses and the receipt of orders for domestic projects in Taiwan.
【United States】
In the U.S., both sales and profit increased significantly year on year. This was mainly due to the steady progress of commissioned projects and favorable sales, as well as revisions to existing contracts due to additional work. New projects have been steadily entrusted by U.S. biotech companies. The company will continue to focus on cultivating the U.S. CRO market and aim for sustainable growth.
【Europe】
In Europe, although the company is achieving sales results by promoting cooperation with the U.S. business, it did not contribute to the increase in sales in the current fiscal year, and sales decreased year on year. In terms of profit, the deficit decreased due to cost-cutting measures. Inquiries have been increasing recently, and the company is strengthening its structure by hiring sales personnel in Europe and focusing on receiving more orders.
【Goodwill balance and remaining amortization period (at the end of FY2024/3)】
| Goodwill | Related intangible assets other than goodwill *2 | ||||
Balance at end of term | Remaining Amortization Period (year) | Annual Amortization *3 | Balance at end of term | Remaining Amortization Period (year) | Annual Amortization *3 | |
Korea | Termination of depreciation in FY 3/19 | Termination of depreciation in FY 3/19 | ||||
Europe*1 | 1,355 | 9-10 | 148 | 9 67 | 3 6.7 | 3 10 |
United states*1 | 2,192 | 10 | 217 | 34 | 3 | 11 |
Total | 3,547 | - | 365 | 111 | - | 24 |
*Unit: million yen
*1 Goodwill generated by the acquisition of Linical Accelovance America, Inc., has been apportioned pro rata to its European subsidiary.
*2 Intangible assets other than goodwill recognized by purchase price allocation.
*3 Figures have been converted at the exchange rate as of the end of the fiscal year ended March 2024.
(3) Change in order backlog
| End of FY 3/24 (A) | FY 3/25 1H | As of November 14, 2024 (B) | Difference from the end of the previous term (B-A)/(A) |
Japan | 3,877 | 3,584 | 3,969 | +2.4% |
United States | 3,221 | 3,188 | 3,345 | +3.9% |
Europe | 3,655 | 3,211 | 3,092 | -15.4% |
Asia | 1,434 | 1,317 | 1,264 | -11.8% |
Total | 12,188 | 11,302 | 11,672 | -4.2% |
*Unit: million yen
In the CRO business, the total amount of clinical trials commissioned to the company, which has an implementation period of one to three years, is determined by the difficulty of the clinical trials due to the number of cases and the target disease. A consignment contract is concluded with the client for this implementation period, and sales are generated according to the contract. In the Contract Medical Affairs Business, the company enters into a consignment contract with a client for a similar period of time, and sales are generated in accordance with the contract.
The order backlog is the balance of orders received for contracted services for which contracts have already been concluded. This is an indicator of sales that will occur over the next one to five years, and is the basis for the company's future earnings forecasts.
The order backlog as of November 14, 2024 stood at 11,672 million yen, down 4.2% from the end of the fiscal year ended March 2024.
In Japan, while the market conditions remained harsh, order backlog increased from the end of the fiscal year ended March 2024 owing to the receipt of several orders for new projects and revisions to some contracts. Meanwhile, in Asia, order backlog decreased from the end of the fiscal year ended March 2024 because the company did not get new orders as it planned due chiefly to the strike by medical doctors in South Korea. Through cooperation between the business in Asia and the businesses in Europe and the United States, the company will tenaciously continue sales activities, for example, by providing overseas biotech companies with proposals to branch out into the Japanese and Asian markets.
In the United States, order backlog rose from the end of the fiscal year ended March 2024 as the company concluded contracts for new projects and revised some contracts for increasing man-hours. Furthermore, Linical has received a number of inquiries for multiple global projects and the like as well as several orders for new projects that are not included in the order backlog mentioned above as it is in the process of concluding contracts for them, and it continues sales activities with the aim of increasing order backlog.In the European region, order backlog has decreased since the end of the fiscal year ended March 2024 as a result of the fulfillment of existing orders and the recording of their sales, despite the receipt of new orders and contract modifications that increased the labor hours involved. Meanwhile, they are receiving orders for new projects through the cooperation with the business in the U.S. and there are multiple projects to be ordered, which are not included in the above order backlog. They will further enhance global synergy in the aspect of marketing, to receive more orders for new projects in Europe, etc. from U.S. enterprises.
(4) Variation in performance in the second quarter (July to September)
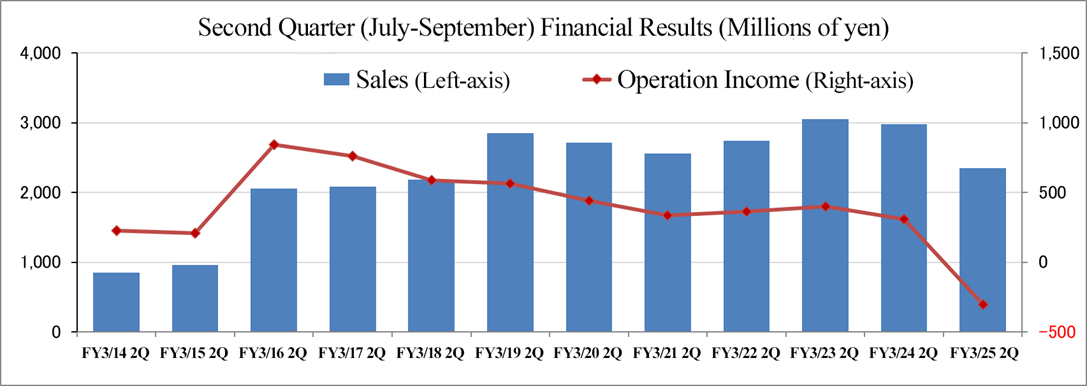
In the second quarter (July–September) of the fiscal year ending March 2025, the company recorded an operating loss due mainly to a sales decline in Japan.
(5) Financial Conditions and Cash Flow(CF)
Financial Conditions
| March 2024 | September 2024 |
| March 2024 | September 2024 |
Cash | 7,465 | 7,213 | ST Interest-Bearing Liabilities | 1,093 | 1,023 |
Receivables and contract assets | 3,463 | 2,869 | Payables, Accrued Expenses | 1,196 | 1,081 |
Advance payment | 1,265 | 1,172 | Advances received | 2,521 | 2,745 |
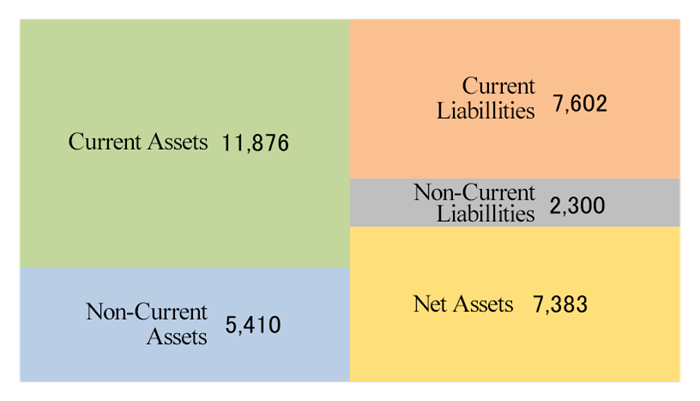
Current Assets | 12,748 | 11,876 | LT Interest-Bearing Liabilities | 1,804 | 1,554 |
Tangible Assets | 518 | 448 | Liabilities | 10,304 | 9,903 |
Intangible Assets | 3,665 | 3,302 | Net Assets | 8,235 | 7,383 |
Investments and Others | 1,607 | 1,659 | Total Liabilities and Net Assets | 18,539 | 17,286 |
Noncurrent Assets | 5,791 | 5,410 | Total Interest-Bearing Liabilities | 2,898 | 2,578 |
* Unit: million yen
* Interest-bearing liabilities=Borrowings + Lease Obligations
*Produced by Investment Bridge Co., Ltd. with reference to disclosed material.
Total assets as of the end of September 2024 stood at 17,286 million yen, down 1,253 million yen from the end of the previous year. On the asset side of the balance sheet, principally, cash and deposits, accounts receivable, advance payment, and goodwill went down while other current assets and deferred tax assets grew. On the liabilities and net assets side, chiefly, short-term and long-term debts, income taxes payable, retained earnings, and foreign currency translation reserves went down, and advance received and deposits received grew. The equity ratio at the end of September 2024 was 42.7%, down 1.7 percentage points from the end of the previous year.
Cash Flow |
|
|
| |
| FY3/24 1H | FY3/25 1H | YoY | |
Operating cash flow(A) | 631 | 541 | -89 | -14.3% |
Investing cash flow(B) | 18 | -12 | -30 | - |
Free cash flow(A+B) | 649 | 528 | -120 | -18.6% |
Financing cash flow | -705 | -662 | +42 | - |
Cash and Equivalents at the end of interim period | 7,316 | 7,213 | -103 | -1.4% |
* Unit: million yen
Regarding cash flows, the cash inflow from operating activities shrank chiefly because the interim net loss before taxes and other adjustments was recorded and the amount of increase in deposits received fell. The surplus of free cash flow decreased mainly because the cash flow from investing activities became negative for reasons such as a rise in expenditure resulting from the acquisition of intangible fixed assets and a drop in income from distributions from investment partnerships. In addition, the cash outflow from financing activities declined mainly because expenditure on payment of lease obligations went down. As a result, the cash position as of the end of September 2024 was down 1.4% from the end of the previous interim period.
4. Fiscal Year ending March 2025 Earnings Forecasts
(1) Consolidated results
| FY 3/24 Act. | Ratio to sales | FY 3/25 Est. | Ratio to sales | YoY |
Sales | 12,307 | 100.0% | 11,468 | 100.0% | -6.8% |
Operating Income | 725 | 5.9% | 250 | 2.2% | -65.6% |
Ordinary Income | 790 | 6.4% | 258 | 2.2% | -67.3% |
Parent Net Income | 338 | 2.7% | 150 | 1.3% | -55.7% |
*Unit: million yen
Sales and profit are projected to drop 6.8% and 65.6%, respectively, from the previous fiscal year.
Linical projects that sales will go down 6.8% from the previous fiscal year to 11,468 million yen and operating income will shrank 65.6% year on year to 250 million yen in the fiscal year ending March 2025.
On November 14, the company downwardly revised its earnings forecast for the fiscal year ending March 2025. While it made steady progress with the existing projects in the United States and generated larger sales than the forecast, it did not receive orders for new projects as forecast in Europe or Japan, and cancellation of a development project in Japan had impact to some extent. In addition, delays in the progress with the existing projects and business negotiations about new projects, which were caused by the large-scale medical strike that started around February in South Korea, affected Linical’s business performance.
While the company controls labor costs, including personnel adjustment, and optimizes SG&A expenses at all the bases, declining sales in Japan and Asia significantly affect its profit. Operating income margin is expected to be 2.2%, down 3.7 points from the previous fiscal year.
The forecast for the amount of dividend paid remains unchanged from 16 yen per share, up 1 yen per share from the previous fiscal year.
Revision to the forecast
| Sales | Operating Income | Ordinary Income | Net Income |
Initial forecast of the company | 12,669 | 1,009 | 1,047 | 697 |
Forecast revised on Nov. 14 | 11,468 | 250 | 258 | 150 |
Amount of increase or decrease | -1,201 | -759 | -789 | -547 |
Rate of increase or decrease | -9.5% | -75.2% | -75.4% | -78.5% |
*Unit: million yen
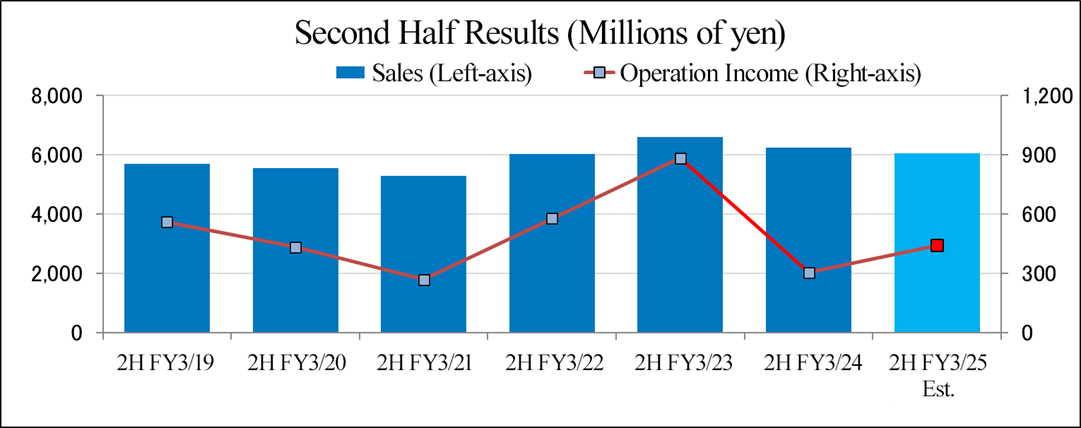
(2) Earnings forecast for the second half of the fiscal year
*Produced by Investment Bridge Co., Ltd. with reference to disclosed material.
For the second half of the fiscal year ending March 2025, the company forecasts that sales will shrank slightly and operating income will grow year on year.
(3) Strategies in each region
Japan and Asia | ◆ In cooperation with the subsidiaries in the U.S. and Europe, Linical will continue tenacious sales activities, for example, by providing European and American biotech companies with proposals to branch out into the Japanese and Asian markets. ◆ Linical will continue sales activities targeting local biotech companies in such countries as South Korea and Taiwan.
|
United States | ◆ Linical receives many inquiries from biotech companies and will continue to strengthen sales activities in order to increase the number of orders it receives. |
Europe | ◆ The centralization of business management in Europe and the U.S. has generated a synergetic effect, allowing Linical to gradually receive orders for new projects. Linical will enhance its sales personnel and focus on getting orders for new projects in Europe. |
5. Conclusions
Their financial results in the first half of the fiscal year ending March 2025 were unfavorable, with sales decreasing 10.5% year on year and an operating loss of 192 million yen. Considering the situation in the first half, they downwardly revised the full-year earnings forecast for the fiscal year ending March 2025 on November 14. The latest forecast of the company calls for a 6.8% y/y decrease in sales to 11,468 million yen and a 65.6% y/y decline in operating income to 250 million yen. This is because sales decreased as they struggled to receive new orders in Europe and Japan as assumed and development projects in Japan were cancelled. Their performance was also affected by the delay in progress of ongoing projects and business negotiations for new deals caused by the large-scale strike by medical doctors that started in South Korea around February of this year. Although the results in the first half of the fiscal year ending March 2025 are unfavorable, there are many topics that make us believe the recovery of business performance from the second half. In the United States, their business performed well in the first half of the fiscal year ending March 2025 and is expected to drive the growth of their business, and there are many new transactions for which they are preparing contracts although they are not included in order backlog. They still receive many business inquiries from biotech companies, and some of the inquiries are about global projects. In Japan, they are expected to receive two large-scale orders next year. In Europe, too, they proceeded with the cooperation with the business in the U.S., so they are about to receive new orders, and there are many new transactions for which they are preparing contracts although they are not included in order backlog. The order backlog as of November 14, 2024 is smaller than that as of the end of the previous fiscal year, although it started increasing at the end of the interim period. It is noteworthy how much they can accumulate order backlog by the end of the current fiscal year, for business expansion from the next fiscal year. In particular, we would like to expect that they will receive large-scale orders from leading biotech companies in the U.S. and leading pharmaceutical companies in Europe.
The company has been concentrating on the fields of oncology, neurology, and immunology, in which they possess strong competitive advantages, since the establishment of the company. In addition to these three fields, they plan to expand their business in the ophthalmic and dermatological fields, where needs will grow in the aged society, and the fields of regenerative medicine and digital medical apparatus, which are new modalities. We would like to pay attention to whether they can receive orders steadily in these fields, which are expected to grow rapidly.
The growth potential in the U.S., which is the largest market in the world, is significant. The expansion of their business in the U.S. is indispensable for the company’s growth. The company aims to expand their business by actively hiring personnel in the U.S. and utilizing M&A. While their business in the U.S. has been expanding steadily, cash and deposits have accumulated and interest-bearing liabilities have decreased, so they are now able to carry out another M&A in the U.S. We would like to keep paying attention to the growth strategy in the U.S. market with expectation.
<Reference: Regarding Corporate Governance>
◎Organization type, and the composition of directors and auditors
Organization type | Company with an Audit & Supervisory Committee |
Directors who are not Audit Committee Members | 6 directors, including 5 outside ones |
Directors who are Audit Committee Members | 3 directors. All of them are outside directors |
◎Corporate Governance Report
Last updated on July 2, 2024
<Basic Policy>
(1) Management Philosophy
Our management philosophy is “To promote the greater wellbeing of all our stakeholders — patients, business partners, shareholders, and employees — we strive constantly to offer professional, high-quality services to support all aspects of new drug development.” We aim to contribute to the development, evolution and diffusion of new therapeutic technologies including new pharmaceuticals, and ultimately to the healthy lives of human beings, by continuously developing and maintaining the knowledge and experience of our executives and employees, as well as the know-how and systems of our organization.
(2) Basic Approach on Corporate Governance
Based on the above management philosophy, our company will contribute to the birth and growth of new disease prevention and therapeutic technologies, including new pharmaceuticals, with our know-how and technologies in pharmaceutical development. As a partner of healthcare companies and medical institutions, including domestic and foreign bio-venture firms, pharmaceutical companies, and medical device manufacturers, our company will contribute to the development of healthcare and meet the expectations of patients and the entire society.
Since our business activities impact people's lives, our executives and employees are required to have high ethical standards as well as expertise. Thus, we thoroughly comply with the Corporate Code of Conduct, including strict compliance with laws. In addition, we strive to improve corporate value and business development by enhancing internal control and ensuring the soundness and transparency of management.
<Regarding the implementation of the principles of the corporate governance code>
Major principles for not implementing and the reasons
Principles | Reasons for not implementing the principles |
[Supplementary Principle 4-1 (2) Medium-term Management Plan] | The Medium-term Management Plan of the company is reviewed by the Management Board, with progress checked and analyzed at each meeting, reviewing the medium-term targets and policies as necessary and appropriate. The Board of Directors approves the Medium-term Management Plan formulated by the Management Board while receiving reports on progress and analysis results, while monitoring and supervising the plan. In December 2021, the company announced a three-year Medium-term Management Plan ending in the fiscal year ending March 2025. In the future, the company will consider revising its targets and policies as required based on progress, disclosing and explaining them together with its vision and management strategies to develop a common understanding with its shareholders and investors. |
[Supplementary Principle 4-2 (1) Compensation System] | In addition to fixed compensation, the company has adopted a performance-based compensation system, a monetary compensation linked to performance over a single fiscal year for directors responsible for executing the company's business. On the other hand, the most executive officers (CxOs) are founding members of the company and already possess a certain number of the company's shares. Therefore, the increase or decrease in shareholder value reflecting medium to long-term performance is linked to the increase or decrease in the value of the shares possessed, which virtually includes incentives similar to medium to long-term performance-based compensation, enabling mutual interest and value-sharing with the shareholders. In this context, no non-monetary compensation, such as share-based compensation linked to medium to long-term performance, has been established. Furthermore, the company will consider necessary revisions to its executive compensation system, including medium to long-term performance-based compensation, in line with changes in the composition of the board of directors, including the appointment of directors other than founding members who will be responsible for business execution in the future. |
[Principle 4-9 Criteria and Eligibility for Independence of Independent Outside Directors] | In addition to the requirements of the Companies Act, the Board of Directors appoints candidates who it believes, based on their knowledge and experience, can actively provide appropriate opinions regarding the management of the company and other matters based on the same objective perspective as ordinary shareholders, after confirming that they substantially meet the criteria of the Tokyo Stock Exchange for determining the independence of independent directors. |
<Disclosure Based on the Principles of the Corporate Governance Code (Excerpts)>
Principles | Disclosure contents |
[Supplementary Principle 2-4 (1) Ensuring diversity in the appointment of core human resources] [Supplementary Principle 3-1 (3) Initiatives for Sustainability]
| The corporate group has formulated a “Sustainability Policy” based on its management philosophy and promotes sustainability management in line with this policy. Sustainability-related initiatives and policies on human resources development, including ensuring the diversity of human resources, and policies on the development of the internal environment, are disclosed in the Annual Securities Report under “2. Policies and Initiatives on Sustainability.” The status regarding the promotion of diversity in the core workforce is as follows. (1) Women The promotion of female managers is progressing at the headquarters in Japan as well as throughout the group, and we will further improve the working environment and provide career development support to develop female leaders at the executive officer level and above, who will play core management roles in the future. 【Headquarters (Japan)】 Female employees/all employees (%) 61.5% in 2024, 62.9% in 2023, 61.6% in 2022. Female managerial positions/All managerial positions (excluding executive officers) (%) 42.6% in 2024, 44.2% in 2023, 42.6% in 2022. Female Executive Officers/All Executive Officers (%) 16.7% in 2024, 16.7% in 2023, 16.7% in 2022. 【Corporate Group】 Female employees/all employees (%) 68.1% in 2024, 68.5% in 2023, 67.5% in 2022. Female managerial positions/All managerial positions (excluding executive officers) (%) 58.6% in 2024, 59.7% in 2023, 56.9% in 2022. Female Executive Officers/All Executive Officers (%) 27.8% in 2024, 31.8% in 2023, 28.6% in 2022. (2) Approximately 50% of the group’s 662 employees (as of March 31, 2024) are locally hired employees residing overseas, and the key positions in the overseas group companies are occupied by highly qualified local human resources. As drug development is getting more and more borderless, we will endeavor to recruit talented human resources with foreign nationality. (3) Mid-career hires: The percentage of mid-career hires among all the employees working at our headquarters in Japan is 48.4% as of the end of March 2024. Mid-career hires account for 100% of the executive officers and 70.2% of the employees who hold managerial positions. For our corporate group as a whole, mid-career hires make up for 71.0% of all the employees as of the end of March 2024. The percentages of mid-career hires among the executive officers and the employees who took up managerial positions are 88.9% and 80.5%, respectively. |
[Principle 3-1 Enhancement of Disclosure of Information] | (i) Our company’s Goals (Management Philosophy, etc.), Management Strategy, and Management Plan Our management philosophy is “To promote the greater wellbeing of all our stakeholders — patients, business partners, shareholders, and employees — we strive constantly to offer professional, high-quality services to support all aspects of new drug development,” and we aim to achieve sustainable growth and to improve corporate value over the medium/long term. In order to achieve this, the company has established a three-year Medium-term Management Plan ending in the fiscal year ending March 2025, which was announced in December 2021. Details of the management strategy and management plan are disclosed in the Annual Securities Report and other documents. (ii) Basic Approach and Basic Policy on Corporate Governance Based on Each of the Principles in this Code Our basic approach on corporate governance is described in “I. Basic Approach” of this report. An overview of our company’s corporate governance, including the above, is disclosed on our company’s website. (iii) Policies and Procedures for the Board of Directors in Determining Remuneration for Executives and Directors Compensation of the company's directors shall be paid within the limits of the total compensation approved by the General Meeting of Shareholders. The Compensation Committee, which consists of 3 or more members, the majority of whom are outside directors, discusses and reports on the decisions and procedures of such policies in consultation with the Board of Directors, to ensure objectivity, transparency, and fairness. Further details are disclosed in “4. Status of corporate governance, (4) Compensation of directors and officers” in Annual Securities Report. (iv) Policies and Procedures for the Board of Directors’ Selection and Dismissal of Executives and Nomination of Candidates for Directors and Executive Officers The appointment and nomination of candidates for executive directors and executive officers are made through the resolution of the Board of Directors based on the candidates' insight and integrity of character appropriate for senior management with regard to compliance with laws, regulations, and corporate ethics, their ability to make decisions accurately and promptly, as well as individual knowledge, experience and ability, taking into consideration the overall balance between the Board of Directors, including outside directors, and the management team as a whole. In addition, the reappointment (or non-reappointment) is resolved by the Board of Directors, based on whether the expected performance and results have been achieved on a constant basis. Candidates for outside directors who are not Audit & Supervisory Committee members shall be appointed and reappointed based on a resolution by the Board of Directors according to the criteria and qualities set out in Principle 4-9. Candidates for directors who serve as Audit & Supervisory Committee members shall be appointed and reappointed based on a resolution by the Board of Directors not only according to the criteria and qualities defined in Principle 4-9, but also by including at least one person who has sufficient knowledge of finance and accounting and obtaining the consent of the Audit & Supervisory Committee while taking into consideration the balance of the Audit & Supervisory Committee so that the committee can audit the business management properly. We will enhance the objectivity, transparency, and fairness of the resolutions made by the Board of Directors on these matters through a process of discussion and reporting regarding candidates by the Nomination Committee consisting of at least three members, of which outside directors constitute the majority. Furthermore, the Board of Directors appoints and dismisses the representative director, president and CEO through a process of discussion and reporting by the Nomination Committee while comprehensively considering such matters as whether our company can respond to changes in the overall business environment, proactively formulate and promote business strategies, and continuously improve our business performance on the premise that appointment and dismissal of the representative director, president and CEO are some of the most crucial decisions to make. Candidates to succeed the representative director, president and CEO are being developed through knowledge training and planned rotations. (v) Explanation of Individual Election and Dismissal of Executives, and Nomination of Candidates for Directors and Corporate Auditors by the Board of Directors Based on the (iv) Above With regard to the nomination of candidates for non-audit committee members and audit committee members as directors, the professional backgrounds and the reasons for the nomination of each candidate shall be stated in the Notice of Convocation of the General Meeting of Shareholders.<Supplementary Principle 4-1 (1) Scope of the delegation to the management> Our company has adopted the executive officer system and established the position of Chief x Officer (CxO) in order to separate supervision of the business management and business execution and swiftly make business decisions. The scopes of matters that the management discusses with the Board of Directors, matters that the management reports to the Board of Directors, and matters that are delegated to the management have been specified in our internal regulations. The Board of Directors makes decisions on matters that are crucial for our business, and decision-making on other business execution that can be delegated in accordance with the law has been delegated to the representative director, president and CEO at our company. |
[Principle 5-1 Policy for constructive dialogue with shareholders] | Through constructive dialogue with shareholders (including institutional and individual investors as potential shareholders), our company aims for sustainable growth in corporate value, which is the common goal of our company and shareholders. Our company is committed to strengthening accountability, is continuously promoting enhanced disclosure of information, and is promoting dialogue with investors in Japan and overseas. The company has continuous, constructive, transparent, fair dialogue regarding business performance, managerial strategies, capital policies, risks, corporate governance systems, etc. with the following method. ・Dialogue with shareholders is led by the Executive Officer CFO. Considering the purpose and effect of the interview, and the attributes of shareholders, the dialogue method is examined thoroughly by the senior management such as CEO and the Executive Officer CFO. ・As for IR, mainly the financial affairs department and the public relations division gather necessary information from relevant sections of the company, prepare reference material and give explanations in an understandable manner, to enrich the dialogue with shareholders.・In addition to the Ordinary General Meeting of Shareholders, financial results briefings, and briefings for individual investors, our company provides opportunities for the dialogue through individual meetings with domestic and overseas institutional investors, by disclosing IR information on our company’s website, including English versions, through phone calls and emails from individual investors, and reflects questions, requests, information on participants at briefings, and survey results in our IR activities. ・Shareholders’ interests and concerns grasped through the dialogue with them are reported to the Executive Officer CFO and the information is utilized for analyzing business administration, discussing how to disclose information, etc.・Concerning IR activities and the dialogue with shareholders, the company manages insider information appropriately in accordance with in-company rules. The quiet period, in which the company refrains from having dialogue about financial results, is from the day after the closing date of each quarter to the date of brief reporting.
【Measures for achieving a business management conscious of cost of capital and share price (under consideration)】 While defining Earnings per Share (EPS) as our business indicator and discloses it via financial results briefing material and our website, our company is currently considering what information to disclose regarding policies, targets, and initiatives. |
This report is not intended for soliciting or promoting investment activities or offering any advice on investment or the like, but for providing information only. The information included in this report was taken from sources considered reliable by our company. Our company will not guarantee the accuracy, integrity, or appropriateness of information or opinions in this report. Our company will not assume any responsibility for expenses, damages or the like arising out of the use of this report or information obtained from this report. All kinds of rights related to this report belong to Investment Bridge Co., Ltd. The contents, etc. of this report may be revised without notice. Please make an investment decision on your own judgment. Copyright(C) Investment Bridge Co., Ltd. All Rights Reserved. |



