| CellSeed (7776) |
|
||||||||
Company |
CellSeed Inc. |
||
Code No. |
7776 |
||
Exchange |
JASDAQ NEO |
||
Industry |
Regenerative Medicine |
||
President |
Yukio Hasegawa |
||
HQ Address |
R-Bldg., Shinjuku 1F, 33-8, Wakamatsu-cho, Shinjuku-ku, Tokyo, 162-0056, Japan |
||
Year-end |
December |
||
URL |
|||
* Stock price as of closing on 2010/8/20.
|
||||||||||||||||||||||||
|
|
* Estimates are those of the company. Data for fiscal years December 2006 to 2008 are non-consolidated.
Mail: CellSeed@cyber-ir.co.jp |
| Key Points |
 |
| Company Overview |
|
CellSeed’s “cell sheet regenerative medicine business” can be compared with the new drug development of pharmaceutical companies, where fundamental research, preclinical research, clinical trial, pharmaceutical approval, national health insurance price listing, and other processes are performed as integral parts of their business cycle. In the “regenerative medical support business”, sales have already been derived from products, but the “cell sheet regenerative medicine business” is still in its preparatory stage and sales have yet to be realized (Sales are expected to be realized during the fiscal year December 2010). <Corporate History>
In May 2001, Yukio Hasegawa, the current CEO, established CellSeed Inc. to develop the regenerative medical business based upon the “cell sheet engineering” as proposed by Professor Okano of the Tokyo Women’s Medical University. CEO Hasegawa holds a Ph.D., was a doctoral researcher at the famous Cleveland Clinic in the United States (Ranked as the number one hospital in its treatment of heart disease), and was the research and development division head at Pharmacia Biotech. Although he was not directly involved with regenerative medicine, Yukio first came across “cell sheet engineering” in 1995, when most medical experts were focused primarily upon genetic therapies and the recognition of regenerative medicine was still low. Therefore “cell sheet engineering” was not highly evaluated, but CEO Hasegawa recognized the huge potential for this technology to fundamentally change medicine. During the initial period after CellSeed was established, research and development was the main activity conducted, and business operations began when an agreement for consigned manufacture of “epithelial cell sheet for corneal regeneration” to be used in clinical trial was reached with Lyon National Hospital in France in January 2007. During September 2007, CellSeed began domestic sales of “UpCell,” temperature responsive cell cultureware that are indispensable in the manufacture of cell sheets.” In October 2008, the Company established CellSeed Europe SARL (current “CellSeed France SARL”) as a subsidiary in Lyon, France to conduct research and development of cell sheet regenerative medical products and applications. In March 2010, CellSeed listed on the NEO market of the JASDAQ, and in June 2010 the Company established CellSeed Europe Ltd. in London, United Kingdom to market and perform sales of cell sheet regenerative medical products in the European market. |
| Fundamental Technologies |
|
(1) “Cell Sheet Engineering” and “Cell Sheet Regenerative Medical Treatment”
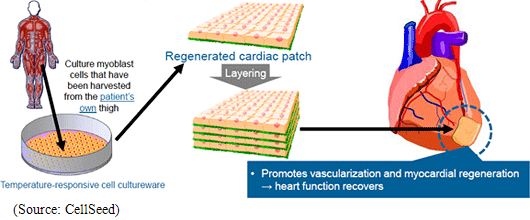
Regenerative medicine is a leading-edge medical technology that utilizes the body’s regenerative capacity by using its own cells to repair various organs and tissues that are suffering from functional disorders and defects. Currently numerous methods are being considered as regenerative medical treatments, but CellSeed has decided to base its fundamental technology upon its “cell sheet engineering” technology in order to pursue the possibilities of regeneration all kinds of tissues and organs. Human cells are constantly undergoing the invisible cycle of death and regeneration. For example, the epithelial cells of the gastrointestinal tract complete the cycle within one to three days, while three to five percent of bone cells complete the regenerative cycle in 90 days. The thing that enables this regenerative process to take place is stem cells (the cells that lie at the root of human metabolism). CellSeed’s fundamental technology is based on “cell sheet engineering” where sheets of stem cells, that are easily accessible from person, are grown (“Cell sheet” creation) and applied to patients to help in the regenerative process. This is the basic treatment process that CellSeed calls “cell sheet regenerative medicine.” 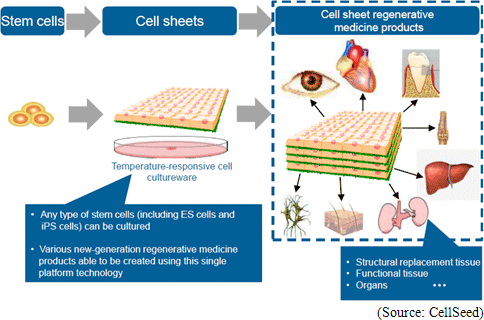 In addition, currently “regenerated cardiac patch” (cell sheet used in heart muscle regeneration), which is undergoing clinical research, is used in the treatment of ischemic cardiac diseases including enlarged heart and cardiac infarction and uses myoblast cells taken from the patient’s thigh to be cultivated in temperature responsive cell cultureware to create cell sheets which are then transplanted to the area to be treated. Working mechanism is assumed that Cytokine and other substances secreted from the transplanted cell patch allow a weakened cardiac muscle tissue to be rejuvenated and for stem cells and other cells to be migrated to help repairing the tissue and to promote regeneration of blood vessels of the affected tissue. Currently in regenerative treatments of cardiac diseases, regenerative cells can be introduced to the affected area by injection to promote regeneration, but the fixation ratio of the injected cells is low. Compared with this, the treatment employing regenerated cardiac patch boasts of a higher fixation rate of transplanted cells and is expected to be a much more effective treatment. (2) ”UpCell” Temperature Responsive Cell Cultureware
CellSeed is currently the only company in the world to manufacture cell sheets using temperature responsive cell cultureware known as “UpCell.” Teruo Okano, a Professor at the Tokyo Women’s Medical University, was the first to propose this “cell sheet engineering” and CellSeed brought this concept into reality with its “UpCell” product. “UpCell” cultureware features a nano-bio interface surface, which consists of a temperature responsive polymer called poly(N-isopropylacrylamide) (PIPAAm) immobilized to the surface at nanometer-scale thickness. Temperature responsive polymers, as the name states, have the ability to alter their molecular structure in accordance with temperature levels and form a bond with a common substrate on the surface of petri dishes using nanotechnology. Cells can be cultivated on the surface of the cultureware at temperatures above 32 degrees centigrade and with appropriate hydrophobic characteristics, and they can be safely separated from the substrate at temperatures below 32 degrees centigrade due to their hydrophilic characteristics. In general, cells excrete adhesive proteins (referred to extracellular matrix) that allow them to form bonds on their own and propagate. The inverse is true where the cells cannot propagate without being able to secrete the adhesive proteins and bonding. In conventional cultivation methods, trypsin and other proteins-like degradative enzymes are used to dissolve and recover adhesive proteins (aside from dissolving adhesive proteins, there was no way of recovering cultured cells). In other words, in conventional methods these enzyme treatments could only be used to recover dispersed cells, and could not recover cells bonded in an organic type of structure. At the same time in applications where temperature sensitive cultivation processes using CellSeed’s temperature responsive cell cultureware are used to collect cells in sheets and extracellular matrixes, they can be easily transplanted to affected tissues and lead to high fixation rates (While dispersed cells also excrete adhesive proteins enabling them to bond to each other over time, their attachment to affected areas remains limited.). Difference between Conventional Cell Cultivation Methods and Temperature Responsive Cell Cultureware “UpCell”
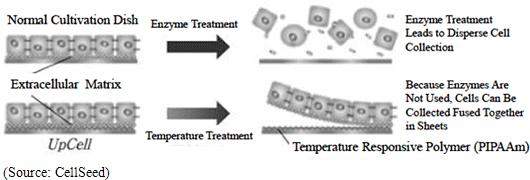 Extracellular Matrix
Supermolecular structures that exist outside of the cell. In addition to filling the space outside of the cell, they also play a structural role and provide a foothold for cells to bond with each other, they also allow cells to propagate and control their division. This is a critical substance to allow cells function as cells.
(3) “Cell Sheet Engineering” Holds the Potential to Fundamentally Reform Conventional Medicine
“Cell sheet engineering” is defined as the use of cells cultivated from temperature responsive cell cultureware and used as “cell sheets” to restructure tissues and organs, and has the following characteristics.
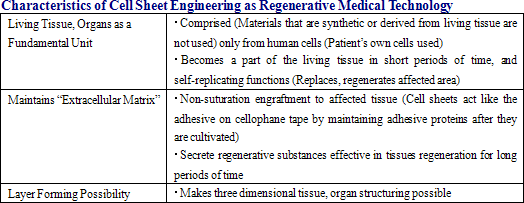 |
| Business Segments |
|
(1) Regenerative Medical Support Business
CellSeed develops and manufactures the temperature responsive cell cultureware and various applications of this product as the fundamental tool in cell sheet regenerative medicine, and provides these products to universities, research institutions and other institutions around the world. This business is not only designed to contribute to earnings, but is also effective in helping to develop partners for the cell sheet regenerative medicine business. A characteristic of CellSeed’s business is the fact that it actively pursues outsourcing and partnerships to make the most efficient use of its limited capital and human resources, and that seeks to leverage the leading edge research results of Tokyo Women’s Medical University through joint research and by outsourcing product development to outside organizations. Furthermore, CellSeed also seeks to avoid having to make large investments in manufacturing facilities by outsourcing certain manufacturing processes. With regards to the marketing function, CellSeed has chosen to provide licenses for the sale of its products to companies such as Funakoshi Corporation, Shimadzu Good Laboratory Component Ltd., Wako Pure Chemical Industries, Ltd., Themo Fisher Scientific Inc. of the United States, which supply services to universities and research institutions that are also users of these products. Furthermore with regards to the iPS cell industry applications project of the New Energy and Industrial Technology Development Organization (NEDO), CellSeed is jointly conducting research on temperature responsive cell cultureware used in iPS cell propagation and cultivation with the National Center for Child Health and Development. (2) Cell Sheet Regenerative Medicine Business
Cell sheet regenerative medical products (Cell sheets) and various applications of these products are sold in this business. Currently CellSeed is conducting joint research and development of five different regenerative medical product pipelines with Tokyo Women’s Medical University, Osaka University, and Tokai University.
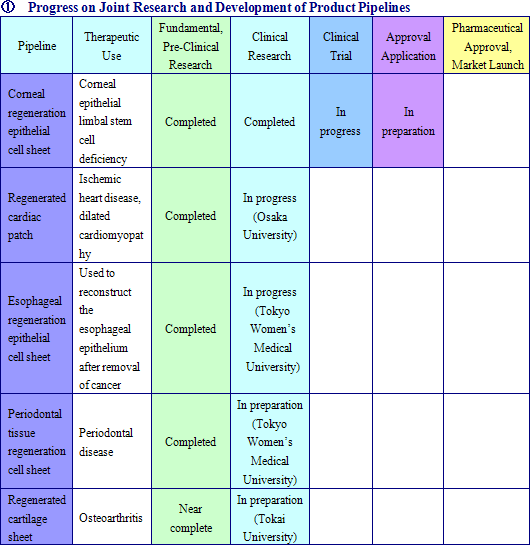 Epithelial Cell Sheet for Corneal Regeneration
Currently undergoing clinical testing of the therapeutic use in corneal epithelial limbal stem cell deficiency in France, epithelial cell sheet for corneal regeneration is now being considered for medical approval in 30 countries and for compassionate use approval in Europe.
Regenerated Cardiac Patch
A product used to treat heart diseases for which radical therapies have not yet been established, it is currently undergoing clinical testing at Osaka University (Currently the first clinical trial patient has been able to remove the artificial heart he was on and has been discharged from the hospital.)
Epithelial Cell Sheet for Esophageal Regeneration
A product designed to reduce or prevent inflammation or esophageal stricture that develops after removal of esophageal cancer. Positive results have been observed in several clinical research tests being conducted by the Tokyo Women’s Medical University where inflammation and esophageal stricture have been prevented in patients to which the product has been used to reconstruct the epithelial layer of the esophagus.
Cell Sheet for Periodontal Tissue Regeneration
Cell sheets are created by using cell of the periodontal ligament collected from the patient’s own wisdom teeth and others, and transplanted to the affected area of the patient to regenerate tissue in cases where moderate to severe tissue damage around the tooth area has occurred.
Regenerated Cartilage Sheet
Cell sheets created from the patient’s own cartilage cells used to treat the affected areas of patients with early to medium term osteoarthritis to regenerate lost cartilage and return the affected area to close to their original state.
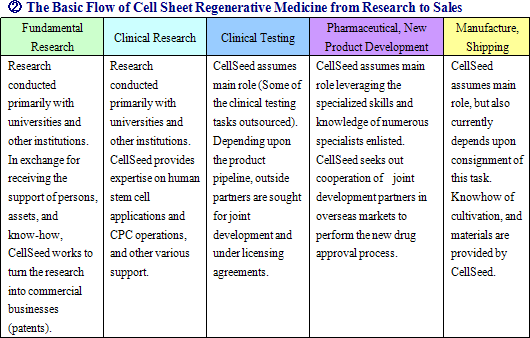 |
| Milestone Disclosure and Business Plans |
 In addition, the reason why clinical testing is being conducted in France and not in Japan is that 1) Odile Damour, head of the cell tissue bank of Lyon National Hospital held a high regard for CellSeed’s technology (Originally Odier made a request to test CellSeed’s technology), 2) France has implemented a highly advanced system for regenerative medicine, and 3) the period required for commercialization is relatively short and the associated costs are low (Because approval will allow for the commercial sale of medical products in 30 countries which are members of the European Medicines Agency, EMA). With regards to reason number 2, “France has a clearly defined guideline for the approval of regenerative medical products, compared with Japan where the industry is still feeling their way around to develop an approval process.” Furthermore approval of medical products in overseas markets makes for a smoother approval process in the Japanese market where the guidelines remain unclear. In addition, Europe allows for compassionate use in instances where there are no other known treatments for patients with severe cases of disease, and where the approval process of the medical approval agency is simpler for these instances of compassionate use. 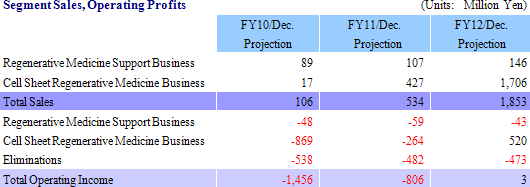 (1) Status of Business Preparation for Epithelial Cell Sheet for Corneal Regeneration in Europe
The clinical testing observations conducted in France for the epithelial cell sheet for corneal regeneration are expected to be completed in August to September, and after an audit process is completed a final report is expected to be issued in October. After this process, approval for commercial use is expected to be submitted to the EMA. And although the approval process takes about one year to complete, compassionate use of the treatment is expected to be launched on a commercial basis in the latter half of 2010 in Europe ahead of official approval. Furthermore after final report on clinical testing is received in France, CellSeed expects to begin preliminary consultations with the Food and Drug Administration of the United States for sales of the same treatment.In addition, CellSeed, in preparation for the medical product approval, has also acquired scientific advice for a second time from the EMA during the current second quarter (April to June) period as part of its process of inquiries for specialized scientific advice from the EMA which began in summer 2009. The acquisition of scientific advice is a reflection of the high regard that the EMA holds for the Company’s epithelial cell sheet for corneal regeneration, and in general about 80% of products for which scientific advice is provided have received official approval from the EMA. 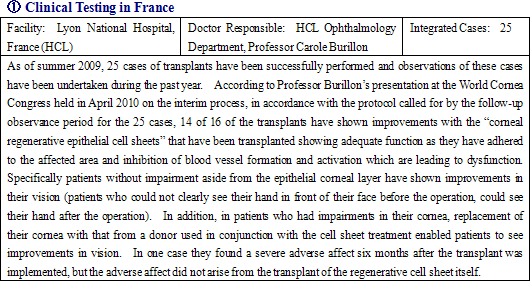 ② Manufacture and Sales
With regards to manufacturing, manufacturing facilities are currently being facilitated at Lyon National Hospital in France along with the technology transfer to TBF Genie Tissulaire (a company involved with the manufacture of tissue engineering products used in cartilage regeneration treatment), the company to which manufacture is being consigned. With regards to sales, CellSeed has reached official agreements with Clonmel Healthcare Limited (Ireland) of the Stada Group in Germany, and GENESIS Pharma S.A. in Greece for them to become the marketing agents. Currently they are in preparatory stages for commercial launch as well as the compassionate use of this treatment, and are developing a network of doctors and institutions which are the target for use of this treatment. CellSeed Europe is responsible for the commercial development of this treatment and will provide the products to these sales agents.
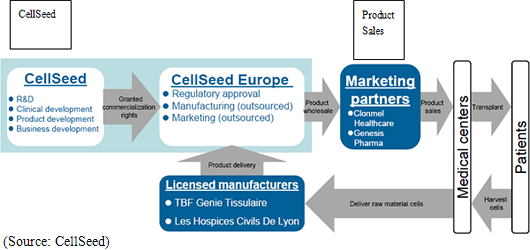 ③ Eurostars Project Grant Acquisition
CellSeed France has been appointed by and will receive support of the Eurostars Project for their jointly conducted work with the Lyon National Hospital, Genesis Pharma, and Paracelsus Medical University of Austria (On a projected basis, of the 3.7 million Euros in total expenses, CellSeed France’s burden of the expenses of between 1.8 to 1.9 million Euros is expected to be received as grants.).Eurostars Project is the public research and development grant program that is operated by EUREKA (A research and development foundation in which various members of Europe participates), that is designed to support the research and development activities of small to medium sized companies who base their headquarters in Europe to realize commercialization of technologies. CellSeed France’s epithelial cell sheet for corneal regeneration business, which has been selected this time, is project as it is close to commercialization, including confirmation of the Good Manufacturing Practice (GMP) in the manufacturing, shipping and other related processes, and undergtaking multicenter-distributed small-scale experiments and rating them over a 30 months period, and final confirmation of these processes using actual products. Furthermore the project was also evaluated for its use by various high profile doctors in Europe (Over 10 doctors anticipated. In addition to promoting compassionate use, this appointment is also expected to help promote the medical approval process and contribute to sales after the treatment comes to market.). (2) Status of Clinical Research for Regenerated Cardiac Patch
In 2007 the medical department of Osaka University started performing clinical research upon the transplant of regenerated cardiac patch treatments for dilated cardiomyopathy. According to the clinical research, myoblast cells taken from the patient’s thigh are used to manufacture regenerated cardiac patches. And in the first case a patient with dilated cardiomyopathy using an artificial heart and waiting to receive a donor heart received a transplant made of over 10 sheets of regenerated cardiac patches layered and not suturing. The patient was able to remove the artificial heart within three months of the transplant because the heart function returned to normal, and another three months later in December 2007 was released from the hospital because the heart was able to maintain its normal function. In addition ischemic heart disease (including cardiac infarction) patients have also become the subject of clinical research started in 2010.
 (3) Joint Research for Regenerative Tendon Treatments Performed with Cleveland Clinic, United States
The Cleveland Clinic is one of the leading comprehensive hospitals in the United States with over 1,000 beds and treating 4.2 million patients per year. The Cleveland Clinic received top ranking in the U.S. News & World Report’s annual ranking of the “United State’s Best Hospitals” for its work in treatments, research, and education, and has been ranked as the number one hospital for its work in heart treatment since 1995. The joint research to be conducted with the Cleveland Clinic is for tendon (tendon connects muscle to bone, and the most well known tendon is the Achilles tendon) regeneration, and more specifically for the applications using cell sheet engineering treatments to produce “tendon cell sheets” used to repair damaged tendon. CellSeed regenerated tendon sheet is expected to fortify its cell sheet regenerative medicine product pipeline, and is viewed as a wide open field because of a few precedent and a lack of competitors. |
| First Half of Fiscal Year December 2010 Earnings and Full Year Earnings Forecasts |
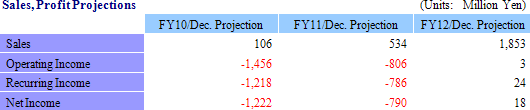
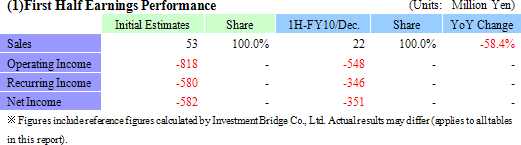 ¥22 Million in Sales, ¥346 Million in Operating Loss
During the first half of the current fiscal, while sales fell below expectations, losses also fell below initial estimates. During the first half, sales of the regenerative medicine support business (sales of temperature responsive cell cultureware) were in line with expectations, but delays of large project deliveries to parties jointly conducting research into the second half and weaker than expected sales in Europe contributed to lower than expected overall sales. The weaker sales in Europe is attributed to stagnant economic conditions in various countries within the region and a rebound from the strong marketing activities conducted in the previous year for the temperature responsive cell cultureware. At the same time, lower than expected sales were offset by lower variable costs due to delays in the occurrence of research expenses associated with corneal regeneration pushing them into the second half and led to lower than expected losses. The corneal regenerative research expenses in Europe are comprised of assistance expenses of start-up contracted manufacturing epithelial cell sheet for corneal regeneration to be paid for joint research conducted with Lyon National Hospital, expenses arising from preparations for the commercialization of the epithelial cell sheet for corneal regeneration, and research assistance expenses paid to other domestic research institutions. Moreover, along with the completion of the publicly subsidized project (NEDO Project), ¥226 million booked as prepayments was reported in grants at the non-operating income. (2) Financial Conditions and Cash Flow
At the end of the second quarter total assets increased by ¥1.539 billion over the end of the previous fiscal year to ¥2.729 billion. And as a result of the funds sourced during CellSeed’s initial public offering in March 2010, cash on hand (Cash and equivalents, and short term government bonds) and net assets increased. At the same time prepayments were realized as non-operating income. Free cash flow grew to a large outflow, due in large part to the acquisition of short term government bonds as part of the Company’s cash management strategy. Because CellSeed has acquired cash on hand of ¥2.5 billion, there appears to be little concern for the cash needs of the company until profits are expected to be realized in fiscal year December 2012.
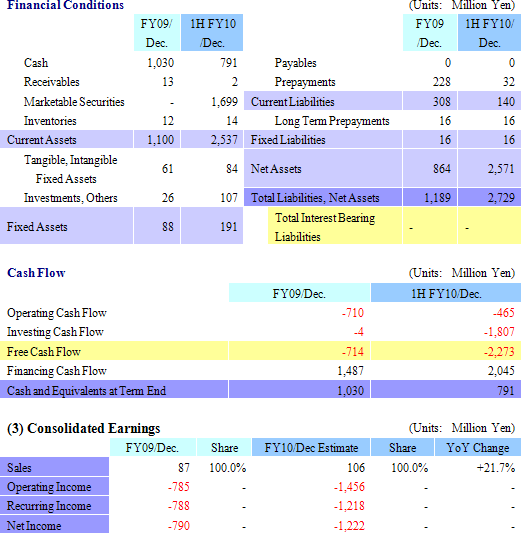 Full Year Earnings Estimates Remain Unchanged: Sales Growth of 21.7%, Recurring Losses of ¥1.218 Billion
In the regenerative medical support business, projects which were delayed are expected to be realized as sales during the second half, and two new products introduced in June and marketing efforts for temperature responsive cell cultureware in overseas markets (particularly the Asia region) are expected to enable CellSeed to realize its full year sales estimate. With regards to profits, expenses arising from research for corneal regenerative epithelial cell sheets in Europe are expected to be booked as expected. And while sales of corneal regeneration epithelial cell sheet in compassionate use applications are expected to rise by ¥17 million compared with the previous term, investments related to the corneal regeneration epithelial cell sheet business are expected to contribute to an expansion in losses.
|
| Conclusions |
|
Compared with these methodologies, regenerative medicine based on “cell sheet engineering” uses the patient’s own human cells and eliminates the ethical issues of ES cells and the risks of iPS cells, and is therefore considered to be the most realistic and safest form of regenerative medical treatment. In addition, the contribution from the epithelial cell sheet for corneal regeneration is expected to lead to realization of profits in fiscal year December 2012. And while the potential for the realization of profits to slip into the following fiscal year December 2013 because unforeseen expenses could arise from the epithelial cell sheet for corneal regeneration, CellSeed’s first major commercial treatment, currently there appears to be little risk regarding the medium to long term direction of CellSeed’s overall business. Therefore investors and patients of difficult diseases can look forward to the business development of CellSeed as the leading company in the field of regenerative medicine. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2010, All Rights Reserved by Investment Bridge Co., Ltd. |



