| Human Metabolome Technologies (6090) |
|
||||||||
Company |
Human Metabolome Technologies Inc. |
||
Code No. |
6090 |
||
Exchange |
Mothers of Tokyo Stock Exchange |
||
Industry |
Service Industry |
||
President |
Ryuji Kanno |
||
Address |
246-2 Mizukami, Kakuganji, Tsuruoka-city, Yamagata |
||
Year-end |
End of March |
||
URL |
|||
*Share price as of closing on the end of December 12. Number of shares outstanding is as of September 30.
ROE and BPS are based on actual results of the previous term end. |
||||||||||||||||||||||||
|
|
*Forecasts are those of the Company. From the current fiscal year, the definition for net income has been changed to net income
attributable to parent company shareholders (Abbreviated as parent net income).
|
| Key Points |
 |
| Company Overview |
|
On the other hand, close attention must be paid to the steady expansion of the metabolome analysis business. Although the company completed its fourth fiscal year since getting listed on December 2013, the total operating CF for the four periods was a surplus 82 million yen. Although it is not a substantial amount, compared to other biotechnology venture, which has nearly a billion yen deficit every term, we can see the advantage of the company with a metabolome analysis business that is steadily profitable. 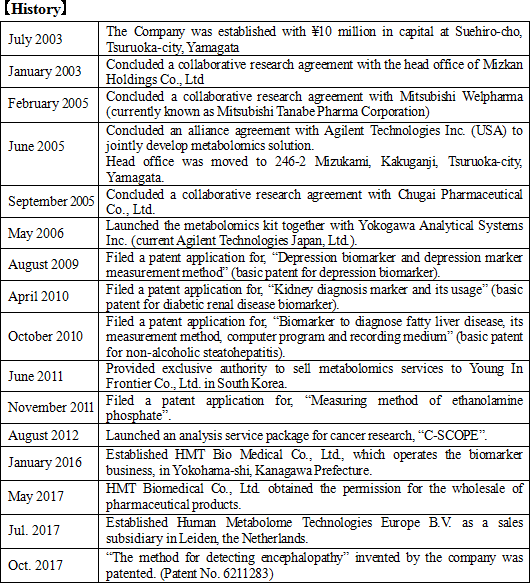 Metabolome analysis techniques had been used for various purposes such as fundamental biological research, development of pharmaceutical products, disease biomarkers, etc., and were expected to expand the demand in the future. Therefore, upon establishment of the CE-MS system, Human Metabolome Technologies Inc. was established in July 2003 to commercialize the method chiefly by Professor Soga, Professor Masaru Tomita, also from Keio University, and the office of Keio University. It was the first venture company originated from Keio University, with a financial support from Keio University's entrepreneurship fund. In 2008, Mr. Ryuji Kanno became the president of the Company. Before taking up this position, Mr. Kanno who was the Vice President & CEO of Agilent Technologies Japan, Ltd., a Japanese subsidiary of Agilent Technologies, a global company that develops, manufactures, sells, and support chemical analysis equipment and electric & electronic measurement equipment in the field of life science., which, had been for some time in business relationships with HMT and Keio University. President Kanno promoted research and development of the Company's core technologies and also began organizing and establishing more specific commercialization process and business models. At the same time, he began preparation for the Company to be listed on the stock market in order to accelerate its growth speed through enhancing the Company's visibility and raising funds for research and development. The Company got listed on the Mothers Section of the Tokyo Stock Exchange in December 2013, ten years after its establishment. "To contribute to people's health and joyful lives through research and development using the up-to-date metabolome analysis technologies for children in the future."  ① Significance of Social Presence
Biomarkers are in vivo substances that are used as indicators to assess the current state of specific diseases. Blood sugar" for diabetes, "γ-GPT" for liver function disorder, and "uric acid" for gout. are the representative examples of well-known and widely used biomarkers.The Company discovered a biomarker for "major depressive disorder", one of the current major social issues, and is developing diagnostic agents to quantify the condition of the disease. Because there is no prevailing method to objectively measure the status of depression, despite the increase in the number of patients with depression, some serious problems are emerging regarding the therapy of depression. For example, patients who would have been cured if they had properly treated are still suffering or in overprescription. If the diagnostic agents using the Company's biomarker become to be widely used, these issues are expected to be solved, and social loss will be subsequently reduced. This social significance cannot be overlooked to understand the Company. ② Excellent Technological Capacities
The Company is highly recognized thanks to the "metabolome analysis technology" which is used to examine complicated behavior of metabolites in human bodies to identify biomarkers. The biomarker for depression is just an example. In Oct. 2017, the biomarker for acute encephalopathy was patented in Japan. With the technology, the Company is expected to identify and develop various new biomarkers in the future. ③ Stable Business Model
The Company's current core business is the "commissioned metabolome analysis business", supporting research and development activities of research institutions and pharmaceutical companies that occupies the greater part of its sales. Sales of FY March 2017 were 913 million (up 29.8%, YoY) and operating income was 501 million (up 66.8%, YoY), showing steady income. On the other hand, the "biomarker business", which is expected to achieve significant growth in the mid- to long-term, is still operated in a small scale and experiencing losses. However, the Company has already established a balanced business model, in which the profit generated from the commissioned metabolome analysis business is invested into the biomarker business for its growth. This model is notable among many bio-venture companies that are suffering from gaining profit. ◎What is Depression?
Depression is a type of mood disorder, and is a brain dysfunctional state for various reasons such as accumulated physical and mental stress. Because the brain is not functioning properly, people in the depressed mood feel negative and low-esteem. This causes a vicious cycle in which people with depression feel more stress for matters that they could otherwise handle. The "major depressive disorder" refers to the state in which depression mood continues even after the sources of stress are removed. In this regard, it is distinguished from adjustment disorders or some anxiety disorders and is considered a brain dysfunction, instead of a mere response to stress. (Note: "Major" of the major depressive disorder means "primary" and does not mean "serious" depression.) ◎Number of depression patients in World and Japan
In 2012, the World Health Organization (WHO) announced that at least 350 million people were with depression which is a mental disorder. Almost 1 million lives are lost due to suicide every year, and over half of them are assumed to be due to depression. According to the "Patient Survey" conducted by the Ministry of Health, Labor, and Welfare (MHLW) every three years targeting health facilities across the country, the total number of patients with mood disorders including depression increased from 430,000 in 1996 to 950,000 in 2011 (2.2x). The "Patient Survey" shows statistical data of the number of patients who visit health facilities. It is known that the consultation rate of patients with depression is low. Thus, according to the MHLW, it is suspected that the actual number of depression patients might be larger. 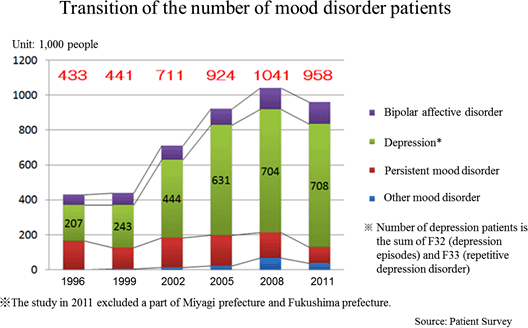 In Japan, economic losses due to depression and suicide are estimated to be about 3 trillion yen. If there were no such losses, GDP would increase about 2 trillion yen (an estimate by the Ministry of Health, Labour and Welfare, 2010). Economic losses around the world are estimated to be about 62 trillion yen in 2002 (Screening for Depression in Adults: A Summary of the Evidence. Ann Intern Med. 2002.). The current economic losses are estimated to be over 100 trillion yen. ◎Treatment of Depression
If someone is diagnosed as having depression, the common treatment is prescription of "antidepressant drugs." The antidepressant drugs can be categorized into several groups such as selective serotonin reuptake inhibitor (SSRI), serotonin-noradrenaline reuptake inhibitor (SNRI), and tricyclic antidepressant. In addition, anti-anxiety drugs or sleep inducing drugs can be used, depending on the symptom. The important thing under drug therapy is that the patients comply with the prescribed amount and frequency after they are informed the effects and side effects. However, patients with depression often reduce the amount or frequency without doctors' permission as they do not feel the symptom to be very serious, they worry side effects, etc. In these cases, the patients do not show improvement as doctors expect, and the doctors prescribe more drugs or change the types of drugs. That would often result in the delay of recovery or excessive administration of drugs, for lack of trust between doctors and patients. Therefore, it is essential to have objective assessment standards quantifying the state of depression or proving the depression has been cured. The biomarker and diagnostic agents of the depression that the Company is currently working on are extremely important for prompt and appropriate treatment of the disease. <What is metabolomics?>
Living organisms including human beings consist of some parts with various functions such as muscles, internal organs, and bones. "Metabolites" such as amino acids, fatty acids, and nucleic acids are common and major components of these organs. Metabolites play crucial roles for entire life activities. Metabolites are provided by food, and are consumed in the process of daily actions such as exercise. They move in a body and cells in accordance with their functions, and are converted into new substances through various chemical reactions, which are called "metabolism". Adjusting body temperature, breathing, moving heart, digesting and absorbing food, transforming old cells into new ones are all operated by metabolism. The "substance conversion" to a new substance is based on a certain flow called metabolic pathway. One of well-known approaches to understand the mechanisms of a human body is "genomics", analyzing genes. Now, automated sequencing and computer analysis of genetic information (DNA base sequences) is available, and nearly all the information in the human genome has already been deciphered. However, much about the relationship between the roles of genes and diseases remains unknown. Recently, more researchers lean towards investigating metabolic profiles, in addition to genetic information coming out of genome analysis, in order to understand the relationships between a human body and diseases. Consequently, research and use of "metabolomics (metabolome analysis)" targeting all metabolites is becoming increasingly popular.  <What are biomarkers?>
A human body puts various vital functions under high-sophisticated manipulation to minimize internal and external influences, and subsequently keeps the body condition stable. That mechanism is called "homeostasis". For example, body temperature and heartbeat may change temporarily, but return to regular ranges. Diseases lead abnormal homeostasis and metabolic status, which are different from healthy conditions. A metabolite, which concentration keenly reflects the status of a specific disease, is called a biomarker. By measuring a biomarker, the current status of a specific disease can be objectively assessed. Blood sugar as a pancreas function indicator, γ-GTP as a liver function indicator, biomarker PSA for prostatic cancer and biomarker CA19-9 for pancreatic cancer are examples of well-known biomarkers. Biomarkers have been studied for a long time in order to monitor the status of diseases. These days, with new methods to analyze multiple substances with higher sensitivity all at once, study results of various new biomarkers have been publishing one after another. Among the biomarkers that are explored through metabolomics technologies are the followings:  Previously, the company allocated the profit from the "metabolome analysis business," which is the current mainstay business, to the investment in the R&D of the promising "biomarker business," and applied the intellectual property obtained through the R&D to the development of medicines and disease diagnosis, with the goal of growing in the mid to long terms. From the term ending Mar. 2017, the company will accelerate the investment in the biomarker business by procuring funds from the outside in order to flourish in the future. Earnings structure and customers of each business are as follows. 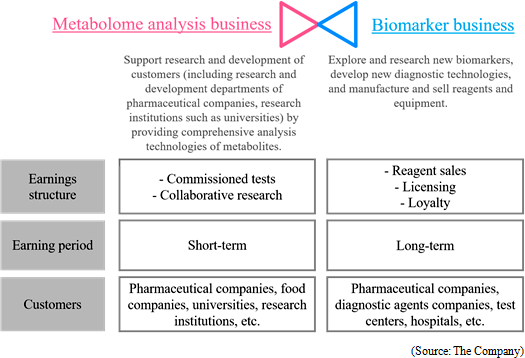 ① Commissioned Metabolome Analysis Business
The Company receives orders from various customers, e.g., private companies such as pharmaceutical companies and food companies as well as universities and public research institutes."Sales were 913 million yen and operating income was 501 million yen for FY March 2017." The scheme of the service is as follows. A customer sends samples to the Company, and then metabolites are extracted from the samples. The extracted metabolites are measured by the CE-MS system, and the acquired data is analyzed. After all, the report is delivered to the customer. The data obtained from the service are used for various purposes; basic biological study, assessment of drug effectiveness and toxicity assessment by pharmaceutical companies, universities, and research institutions, analysis of fermentation process and functional evaluation of functional foods by food companies. The data is contributing to the progress of research and development activities of customers. In recent years, not only healthcare, food, but also interest towards health-related companies is emerging rapidly. ◎Deployment in the Overseas Market
In order to distribute the commissioned metabolome analysis service in Asia, the Company concluded an exclusive sales authority agreement with Young In Frontier Co., Ltd. in South Korea in June 2011. Moreover, the Company hired a sales representative in charge of Asia-Pacific area to develop Asian market including Singapore and Hong Kong, outside of South Korea. Furthermore, in order to expand its business in the North American market, it also established its sales subsidiary, Human Metabolome Technologies America, Inc., in October 2012 at Cambridge, Massachusetts, USA, home to many medical research institutions.In order to further accelerate overseas development, in May 2017, the company established a subsidiary (sub-subsidiary) "Human Metabolome Technologies Europe B.V." in Europe (Netherlands) through HMT-A. ◎"C-SCOPE" -a service package of the commissioned metabolome analysis service for cancer study
In August 2012, the Company launched "C-SCOPE", a service package of the commissioned metabolome analysis service well organized for cancer study. C-SCOPE was developed to respond to the needs; they would like to measure concentrations of specific metabolites changing inside cancer cells with higher sensitivity and higher accuracy. Its technologies are based on unique and efficient metabolites extraction method from cancer cells and highly sensitive analytical system.Cancer is the number one cause of death in Japan since 1981 and occupies about 30% of the recent total causes of death. According to the MHLW, the costs for cancer research are increasing year by year; in 2012, ¥35.7 billion were spent. It is an urgent task for many pharmaceutical companies to develop effective new anticancer drugs. The "Warburg effect" is the phenomenon that most cancer cells have glycolytic rates of several to dozens of times to that of normal cells. Although this effect was proposed as far back as over 80 years ago, the research on the effect made little progress since there was no method to comprehensively measure metabolites back then. Thanks to the dramatic advancement in metabolomics technologies in recent years, development of anticancer drugs which act as metabolic inhibitors are underway. The Company's metabolome analysis technology based on the CE-MS system is considered as one of the effective analysis systems applicable at each stage of cancer research; from the basic study of cancer biology to clinical application in the process of anticancer drugs development. ②Biomarker Business
The Company considers the business related to biomarkers, which play an important role in occasions such as early diagnosis or monitoring treatment effects, as a driver for future growth, and is proceeding with biomarker discovery and development of clinical test drugs through collaborative research and development with universities, pharmaceutical companies, and diagnostic drugs companies. "Sales was million 0 JPY and operating loss was 198 million JPY for FY March 2016" The Company develops new diagnostic methods by using biomarkers, which were acquired through its own R&D, or biomarkers introduced from outside. Additionally, through the process of product development and clinical development, the Company produces and sells in vivo diagnostic drugs and diagnostic equipment. The sales in this business are composed of cooperation money for R&D from pharmaceutical companies in cooperative research, milestone revenues, and loyalty gained from the sales of the drugs when they are commercialized. ◎Intellectual Property Policy
The personnel in charge of intellectual property and contracts works on patent application and requests for examination of all projects, and are in close collaboration with the patent attorneys of the Company and its collaborative research institution. They are also responsible for negotiation concerning collaboration research agreements and development of agreement documents. The Company attempts to maximize its rights when obtaining a patent of newly discovered biomarkers.Since the scope of the rights differs depending on the marker, the Company generates patent application documents in a way that would cover a wide scope of the rights such as chemical structure of the biomarker, usage for diagnostics and drug development, detection method and measurement equipment. Furthermore, the Company makes it a principle to file international patent applications in accordance with the Patent Cooperation Treaty, in anticipation for the future license agreement and market based on the information of clinical test drugs, test equipment companies and pharmaceutical companies of various countries. As of June 2017, the "basic patent" on the method of biomarkers of depression diagnosis etc. has been registered in Japan, the US and China (Europe has been filed). The patent on the method of measuring ethanolamine phosphate (PEA) has been registered at all four stations in Japan, the US, China and Europe. ◎Example of Biomarker Business: Depression Biomarker
The Company especially focuses its research and development on a) the central nervous system disorder such as depression (e.g. mood disorder and mental disorder) for which there are few objective diagnosis methods b) diseases that have become social problems such as metabolic syndrome, including hepatitis and diabetes, and their related diseases. Its current focus is the biomarker for depression.Diagnosis of major depressive disorder is conducted using the diagnostic standards provided by the American Psychiatric Association or the standards of the World Health Organization (WHO). However, both of them largely reflect the subjective view of the doctor or patient, and unlike other diseases, no diagnostic method has been established on the basis of objective indicators. The Company conducted collaborative research with the National Center of Neurology and Psychiatry, and discovered a blood biomarker of major depressive disorder. Blood samples were collected from approximately 30 patients and 30 healthy people, and the blood components were compared through metabolome analysis using the CE-MS system. As a result, the patients with major depressive disorder showed lower concentration of (PEA) in their serum. Through further analysis, it was found that a) PEA is a specific biomarker for major depressive disorder, and b) PEA level will return to the healthy standard range when MDD is treated. 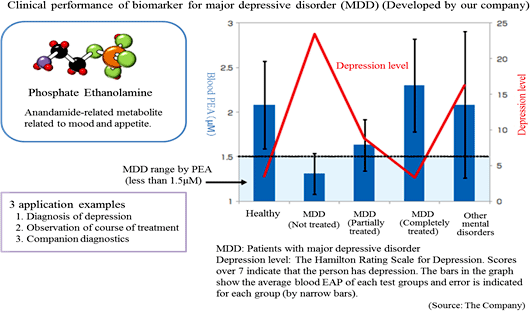 *Companion diagnostics: Refers to clinical tests to predict effects and side effects of pharmaceutical products before administration. By checking the responses of individual patients towards a drug before treatment, more effective drug administration can be provided.
Subsequently, the Company developed a technology to measure extremely low-concentrated PEA in the blood, which was not possible to detect with the conventional enzyme method. Based on this technology, the Company developed the "PEA test kit using the enzyme method" (β-version) in 2016. It is of extreme significance that the Company succeeded in the development of a "depression biomarker test kit" (β-version). "Realization of an inspection method that can be processed cheaply and massively" and "Establishment of a technical foundation that can supply reagent kits worldwide" will make it possible to supply PEA tests to 350 million people with depression worldwide. As a result the company's significance of social existence has become even bigger. In addition, it has become possible to shift to a new business development phase that considers concrete markets, product specifications, distribution channel construction, business size, etc.  ◎Identification of Disease Biomarkers
The Company uses the following three connections and systems for identifying biomarkers in order to expand biomarker development pipelines.
<Connection with the Customers for Commissioned Analysis or Collaborative Development>
The Company accepts requests from universities and companies for the tests for finding biomarkers. The Company also receives proposals for collaborative development before or after the tests. Currently, a collaborative development project for diabetic nephropathy biomarkers is ongoing.
<Direct Proposal to Researchers and Physicians>
The Company's researchers directly propose research plans for the development of disease biomarkers to researchers or physicians, and establish collaborative study agreement with the institution based on approvals from the collaborative researchers or physicians. The target diseases are chosen according to the number of patients, compatibility to the analysis technologies of the Company, degree of social contribution, and necessity of biomarkers. In addition to major depressive disorder, the Company is working on the development of biomarkers for non-alcoholic steatohepatitis and fibromyalgia.
<HMT Research Grant for Young Leaders in Metabolomics>
The Company offers a grant (HMT Research Grant for Young Leaders in Metabolomics) to graduate students to disseminate the usefulness of metabolomics in the society and nurture young researchers. From the research themes submitted from graduate students across the world, the Company chooses excellent proposals, and supports their research by awarding metabolome analysis service without a fee. Fourteen students have been awarded in the last 4 years. Some of these study results actually led to the identification of biomarkers and evolved to collaborative study with the Company, for example, infectious disease-related encephalopathy biomarker.
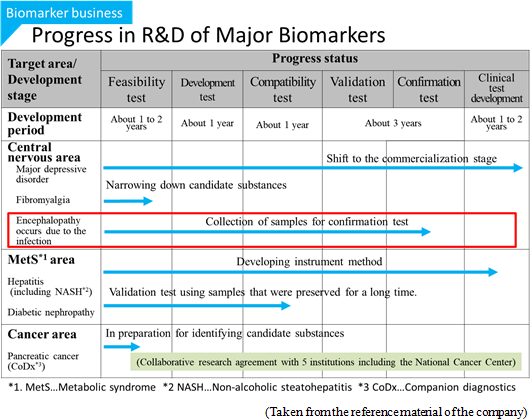 Furthermore, in Oct. 2017, the "method for detecting encephalopathy" was patented in Japan, as the company and Nagoya University jointly applied for the patent. Encephalopathy occurs due to the infection with viruses, bacteria, or the like, and induces the impairment of consciousness, spasm, abnormal behavior, hallucination, etc. Especially, acute encephalopathy, such as influenza-associated encephalopathy and HHV-6 encephalopathy, occurs to children, and in many cases, it progresses rapidly and becomes severe. Accordingly, it is necessary to diagnoze them early and offer appropriate treatment immediately. In collaborative research with Nagoya University, the company discovered a candidate biomarker for detecting acute encephalopathy, and made it patented. |
| First Half of Fiscal Year March 2018 Earnings Results |
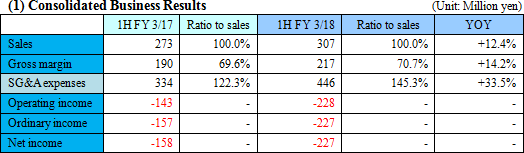 The company still incurred loss due to the increase in investment for growth.
Sales were 307 million yen, up 12.4% year on year. As metabolome analysis became popular mainly in the food product field, the company received large-scale orders from pharmaceutical companies. The sales of the metabolome analysis business marked a record high for the cumulative second quarter. Operating loss was 228 million yen, indicating the augmentation of loss from the same period of the previous year. For the metabolome analysis business, the company upgraded its equipment and organizational structure for launching a new analysis plan. For the biomarker business, the company keeps investing in the commercialization of depression biomarkers. 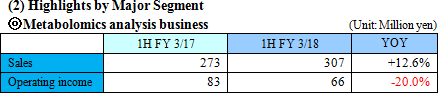 Profit dropped 20.0% year on year, because the company invested in equipment for launching a new analysis plan and concentrated on the fortification of its organizational structure. The amount of orders received healthily increased 20.9% year on year. (Topics) ◎ A subsidiary in Europe started business operation. "Human Metabolome Technologies Europe B.V. (HMT-E)," which was established in the Netherlands for cultivating the European market for the metabolome analysis business, started operating business in July 2017. The company aims to accelerate the overseas expansion of the metabolome analysis business by utilizing the know-how and resources obtained through the process of establishing HMT-A, a subsidiary in the U.S., and doing business based on the cooperation between subsidiaries in the U.S. and Europe. ◎ Pursuit of a marker for detecting Parkinson's disease early As a research supporter in this project, the company carried out the metabolome analysis of metabolites in blood, and confirmed that patients of early-stage Parkinson disease have smaller amounts of 7 kinds of long-chain acyl-carnitines in blood than normal people. It can be expected that the precision of early detection of Parkinson disease will be improved and it will become possible to diagnose Parkinson disease before its onset with a simple blood test. 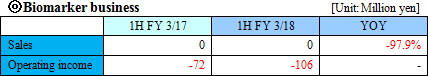 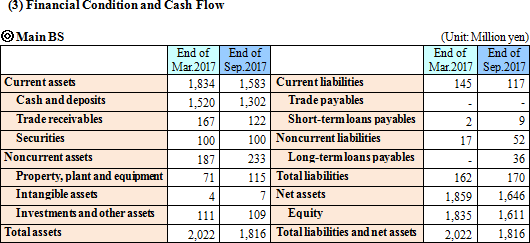 As a result, equity ratio declined from 91.4% at the end of the previous term to 89.5%. 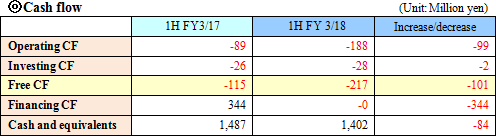 Investing CF is nearly unchanged, and the deficit of free CF grew. Financing CF became negative, because the income from the issuance of shares for the exercise of share acquisition rights, which was posted in the same period of the previous year, was not posted this year. The cash position degraded. |
| Fiscal Year March 2018 Earnings Estimates |
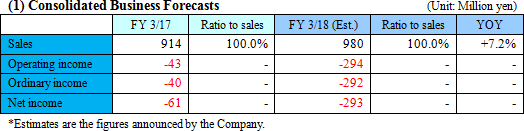 No revision to the earnings forecast. The metabolome analysis business is expected to remain healthy, increasing sales. The upfront investment in the biomarker business will expand, augmenting loss.
There is no revision to the earnings forecast. Sales are estimated to be 980 million yen, up 7.2% year on year. The performance of the metabolome analysis business is forecasted to remain healthy. As the company will actively invest in the two businesses, loss will augment.
(Targets of intensive investment)
* Metabolome analysis businessThe company will invest 150 million yen in equipment, and start new analysis services, including the services for searching highly-sensitive biomarkers with CE-OrbitrapMS. Metabolites are classified into water-soluble and fat-soluble ones, and the company's analysis of water-soluble metabolites is highly evaluated. The company analyzes fat-soluble metabolites, too, but it still does not offer a service specializing in lipid analysis. This term, the company is developing new services for lipid analysis, for the purpose of expanding business in this field. * Biomarker business The company will invest 315 million yen in production development for releasing reagents for PEA measurement and research by the end of this term. (2) Activities in this term (from the previous report)
The environment surrounding this business is changing considerably. ◎ Metabolomics analysis business Especially, metabolomics has evolved from academic technology for conventional universities, laboratories, etc. to industrial technology. In these circumstances, the companies in the health-conscious market, including new healthy food products, such as functional foods, sports, food products, sleeping, and stress, have interests in the effectiveness of metabolomics, which grasps the health conditions of human beings, and new markets are emerging. In the field of medicine development, metabolomics is considered effective for researching intestinal bacteria, early detecting neuropsychiatric disorders, such as dementia and Alzheimer's disease, diagnosing them, developing treatment methods, and putting medical technologies, including medicines for refractory diseases, into practice. In such a favorable trend, the company will implement the following measures this term, and steady growth is expected. 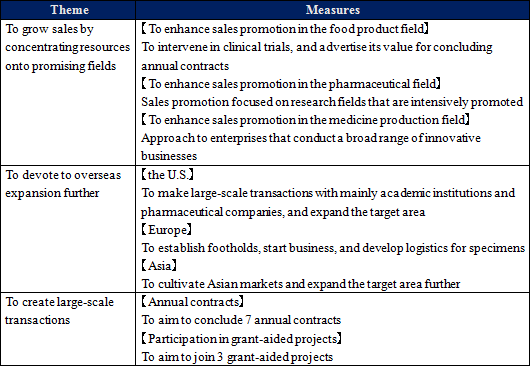 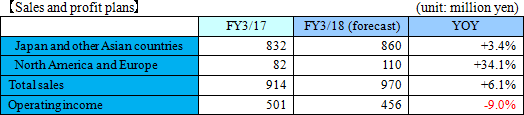 ◎ Biomarker business
The company assumes two kinds of PEA measures: "the measurement using a kit of measurement reagents and versatile automatic equipment for biochemical testing at large hospitals and clinical test centers" and "the measurement using a measurement reagent cartridge and POCT measurement equipment at general internal medicine and mental clinics." For the former, it is necessary to develop a kit of measurement reagents compatible with automatic equipment. For the latter, it is essential to develop POCT measurement apparatus and measurement reagent cartridges indispensable for clinical measurement at co-researching institutions. Following acivities are conducted in each realm now. *Product development The development of measurement reagent kits compatible with automatic equipment leads to the foray into the health checkup market, which is much larger than the market of diagnosis. As for POCT measurement equipment, the company will develop a prototype (before mass production) by the end of this term. ( Real-time test using a compact analyzer and a swift diagnosis kit in a medical scene. It includes all kinds of clinical tests conducted at a hospital laboratory or a place other than an outsourcing center. A wide range of testing places and application methods can be assumed. Since medical staff conduct POCT beside a patient, testing time can be shortened and the patient can feel familiar. These merits are said to contribute to swift, appropriate diagnosis and nursing, prevention of diseases, health promotion, and the improvement of quality of life (QOL) of patients. *Clinical development
In order to apply for the approval for the production and sale of pharmaceutical products, it is indispensable to obtain and analyze a sufficient volume of data. To do so, it is necessary to collect clinical data from medical doctors and hospitals, which can be said to be third parties for the company. Accordingly, the company will promote many facilities and medical doctors to conduct a PEA measurement test, and clarify the gap from their hypothesis and the variation of doctors' judgements. In order to achieve early full-scale commercialization, it is indispensable to specify a condition such as "a measurement reagent only for diagnosing depression with the decline in the PEA level in the blood" rather than "diagnosing all kinds of depression," after the feasibility tests by many facilities and medical doctors. In addition, it is considered important to do academic research for backing up clinical studies. Accordingly, the company will elucidate the mechanism for the drop in the PEA level and study the effects of the administration of antidepressants by using model animals in collaboration with Japanese universities, and clarify the mechanism for the PEA generation in the brain by using biochemical methods in cooperation with a U.S. national institute, while considering future global business operation. *Pharmaceutical affairs
In Feb. 2017, the company obtained the approval for the manufacturing and sale of in-vitro diagnostic agents. Then, it became possible to outsource the manufacturing of in-vitro diagnostic agents to an enterprise that has "a license to produce in-vitro diagnostic agents" and sell the in-vitro diagnostic agents produced by outsourcing. This is required for obtaining the approval for the sale of the company's biomarker as an in-vitro diagnostic agent in accordance with the Pharmaceuticals and Medical Devices Law.In May, the company received the permission for the "wholesale of pharmaceutical products." If the sale of the company's biomarker as an in-vitro diagnostic agent is approved in accordance with the Pharmaceuticals and Medical Devices Law, it will become possible to directly supply this in-vitro diagnostic agent to a broad range of medical institutions, including hospitals, clinics, and clinical test centers. Like this, the company laid the groundwork for future pharmaceutical business. This term, the company will start consulting with Pharmaceuticals and Medical Devices Agency about the application for the approval for the production and sale of pharmaceutical products, and discuss concrete exit strategies. *Business development
The company will proceed with the production of PEA measurement reagent kits (for research), and release them targeting mainly research institutes in Japan in the second half. The basic patent for the PEA enzyme method is pending only in Europe, but the company considers it to be registered soon.  |
| Progress of Biomarker business |
 (1) Start of a new management system for commercialization
In order to accelerate the commercialization of depression biomarkers, the company established a new management system of HMT Biomedical Co., Ltd. The new management is composed of members who possess plentiful experience and knowledge for the commercialization of biomarkers, as tabulated below. 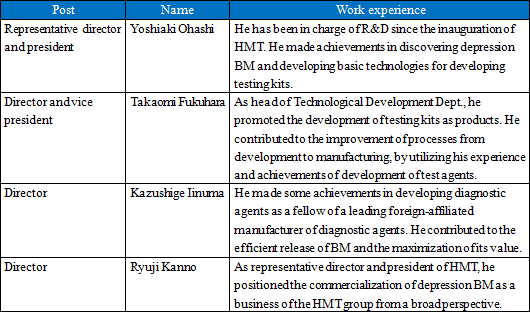 (2) For the sale of a kit of reagents for PEA measurement (for research)
In Oct. 2017, it was announced that the company established a technology for mass-producing enzymes used for measuring depression biomarkers through the collaborative research with Toyobo Co., Ltd. (3101, the first section of TSE). Toyobo is a leading enterprise boasting the second global market share in the production on industrial enzymes, and supplies enzymes continuously for the kit of reagents for PEA measurement (for research) sold by HMT. HMT will start selling products to research institutes in the second half, as it became possible to develop a system for stably supplying reagents for research, which are indispensable for testing kits, and extract improvement points in the application for permission for new medicines by collecting the voices of customers. In addition, Toyobo is scheduled to support the development of pharmaceutical products for in-vitro diagnosis of depression as the next step. |
| Interview with President Kanno |
|
Q: "Please tell us first about the current situation and future outlook of the metabolome analysis business."
A: "Its sales have been growing by double digits as the market in Japan has been expanding. Our foothold for cultivating the huge overseas market has been completed."
Its performance remains healthy. From now on, the double-digit growth is expected to be continued for the foreseeable future.Our technology had been used mainly in academia, including universities and laboratories, but in recent years, the adoption by private enterprises has been increasing. Especially, food companies have strong needs for and keen interests in the effectiveness of our products for "quality control" and "value verification." Private enterprises allocate a large budget to good ones. As the value of metabolome analysis is being clearly recognized, its market is expected to grow further. In a nutshell, clients' evaluation on our company can be expressed by "reliability." Since the inauguration of our business, we have dealt with over 4,000 analysis cases. This achievement is unrivaled in Japan, and top-class in the world. Over 4,000 samples which is rich in variety provide us with valuable "big data," and the "support for metabolome data analysis" utilizing the data made a good start. On the other hand, our company's presence and market share are still insignificant in the global market, which is said to be over 10 times larger than the Japanese market. Accordingly, we founded a corporation in the Netherlands, and developed business footholds in Japan, the U.S., and Europe. Although our market share is small, we plan to accelerate the overseas expansion of our metabolome analysis business and realize discontinuous growth by utilizing our know-how and resources acquired through the process of establishing HMT-A, a subsidiary in the U.S., and conducting business based on the cooperation between subsidiaries in Europe and the U.S. Q: "How is the biomarker business?"
A: "As we took a significant step in the previous term, we have steadily laid the groundwork for early commercialization."
In the FY ended March 2017, we developed the kit of reagents for measurement for research and POCT devices, established for a system for clinical development, obtained the permission for manufacturing and sale of pharmaceutical products, and made the PEA measurement method patented in the U.S., China, and Europe, taking a significant step. This term, we have steadily laid the groundwork for early commercialization, by obtaining the permission for wholesale distribution and developing a mass-production system for stably supplying indispensable enzymes for reagent kits in cooperation with Toyobo. In addition, the competent management was organized in the subsidiary HMT Biomedical, as a significant step. Commercialization will be realized more speedily, if HMT Biomedical rather than the parent company, which operates mainly the metabolome analysis business, conducts development and negotiation with authorities, as HMT Biomedical has very competent personnel specializing in diagnostic agents. The company will first operate business firmly for attaining the goals in fiscal 2017. 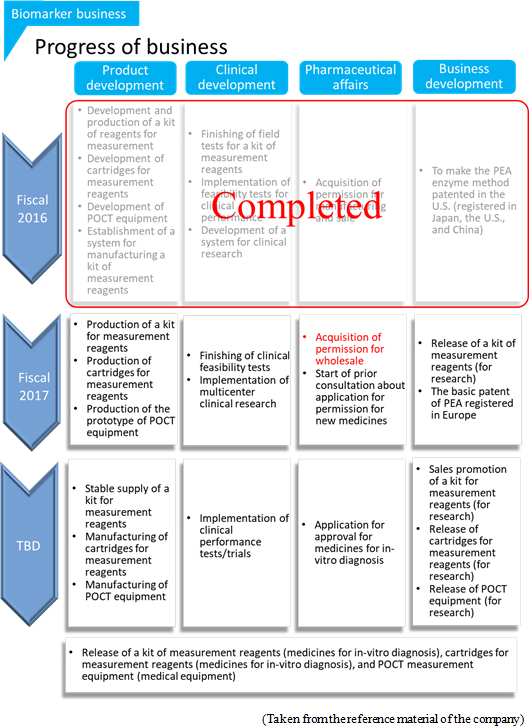 In Oct. 2017, the domestic patent of an acute encephalopathy biomarker was registered. This is also a big step. Most patients of acute encephalopathy are infants, toddlers, or children (0 to 12 years old). They first suffer from the symptoms of a cold, and some patients have permanent damage, such as impaired consciousness, spasm, and abnormal behavior, several hours or days later, or die of failure of multiple organs. The mechanism for its onset is unknown, and the method for diagnosing it is still to be established, but if early diagnosis is actualized, it may be possible to conduct appropriate medical treatment at an early stage, and would contribute to the development a testing kit following the kit for major depressive disorder. It was also found that it is possible to develop biomarkers efficiently by utilizing the knowledge and know-how accumulated through the development of biomarkers for major depressive disorder. We will utilize the valuable lessons from experience and enrich our pipeline. We procured funds through listing in the stock market, recruited excellent personnel, and proceeded with development steadily. In the spring of next year, 6 new graduates will join our company for the first time after listing. All of them have a doctoral degree. We held a ceremony for welcoming prospective employees, and these youngsters are so entrepreneurial that they said, "I am certain that I will contribute to corporate growth" and "In HMT, my ambition could be realized." Then, I feel reassured. We would like to grow considerably while recruiting new graduates every year and create a culture unique to HMT. Q: "Lastly, please give a message to shareholders and investors."
A: "As all workers of our company join hands to meet the expectations from shareholders and investors through the significant growth of the two businesses, we would appreciate your support."
It's time for our corporate group to consider the approval for medicines and insurance coverage regarding the development, manufacturing, and sale of a PEA testing kit. Accordingly, we will refrain from disclosing some information regarding the development of the kit and the preparation for the manufacturing system. We would like you to understand that this means that the commercialization of biomarkers has progressed smoothly and we have entered an important stage. In addition, we have nearly completed a system for taking advantage of the expansion of the global market of metabolome analysis with our unique technology. As all workers of our company join hands to meet the expectations from shareholders and investors through the significant growth of the two businesses, we would appreciate your support. |
| Conclusions |
|
Some goals for product development, clinical development, pharmaceutical affairs, and business development are still to be attained. We would like to pay attention to each progress in this term. |
| <Reference: regarding corporate governance> |
 ◎ Corporate governance reports
Updated on July 5, 2017<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)> It is mentioned that "Our company follows all of the basic principles of the Corporate Governance Code." Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation for investment. The information and opinions contained within this report are made by our company based on data made publicly available, and the information within this report comes from sources that we judge to be reliable. However, we cannot wholly guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2018 Investment Bridge Co., Ltd. All Rights Reserved. |




