| EIKEN CHEMICAL (4549) |
|
||||||||
Company |
EIKEN CHEMICAL CO., LTD. |
||
Code No. |
4549 |
||
Exchange |
Tokyo Stock Exchange, First Section |
||
Industry |
Pharmaceuticals (manufacturing and sales) |
||
President |
Morifumi Wada |
||
HQ Address |
7 Yamaguchi building, 4-19-9 Taito, Taito-ku, Tokyo 110-8408, Japan |
||
Year-end |
End of March |
||
URL |
|||
*Share price is as of the end of June 15. The number of shares issued is from the latest financial settlement report (excluding treasury shares from the number of shares issued). ROE and BPS are based on actual results at the end of the previous term.
|
||||||||||||||||||||||||
|
|
*Estimates are by the Company. The definition for net income means net income attributable to owners of parent.
|
| Key Points |
 |
| Company Overview |
|
It offers many products that occupy high market share including fecal immunochemical test that occupy about 60% of the domestic share, Urinalysis test, Microbiological test and so on. Its unique gene amplification technology, "LAMP", is highly recognized in the world. With the fecal immunochemical test reagents, urinalysis test strips and LAMP, EIKEN is aiming to become a global corporation.
"Management Vision": EIKEN group is dedicated to leveraging expertise as a medical testing pioneer in order to increase corporate value by protecting the health of the public with products and services that customers can trust. "Motto": We EIKEN provide trustworthy quality, and develop with technology. EIKEN group formulates "EIKEN WAY" as its attitude toward each stakeholder, centering these philosophy, vision and motto. 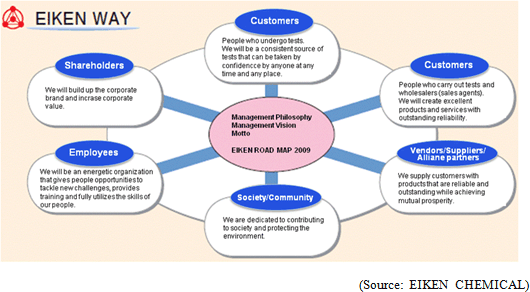 <Domestic Market>
The market scale of clinical reagents is about 348.5 billion yen and 591.2 billion yen (including research reagents and diagnostic devices) as of 2015 (survey by the Japan Association of Clinical Reagents Industries, or JACRI). In order to control rising medical costs, the Japanese government is focusing on preventive medicine such as special health check-ups (metabolic check-ups) and cancer screenings. It is expected that this, along with the aging population, will lead to an increase in the number of samples (number of specimens). Some negative factors include the impact of population decline as a result of decreasing birth rates and revision of medical treatment fees (reduction). However, the trends of laboratory test fees which had been subject to revision of insurance (medical laboratory test fees) show that, even though they were cut by some 40% from 1997 to 2006, the fees have been stable or only slightly reduced after 2007. (Laboratory test fee in fiscal 2016: -0.4%) This is a result of advocacy on the importance of preventive medicine and clinical diagnostics by the entire industry including Eiken. Thus, in the mid to long term, the domestic market is expected to slightly grow by 2% each year. Out of the 129 member companies (as of November 2016) of JACRI mentioned above, about 80 are manufacturers, and there are about 15 companies with over 10 billion yen in sales. Most of them are small to medium sized companies. Because the test items of diagnostic tests range widely, each company has its own field of strength, and business segregation is already established in the industry. As a result, collaboration, such as supplying raw materials and products from other companies and manufacturing and selling them, is often observed. Against such a backdrop, the market is modestly growing. Therefore, there is currently no apparent trend of weeding out uncompetitive corporations. <Overseas Market>
The global clinical laboratory test reagent/device market is estimated to be US$ 56 billion and, by region, the market is occupied by the USA at 42%, followed by Europe at 33% and Asia/Pacific at 22%. The overseas market is over ten times larger than the domestic market. In developed countries, the number of tests is increasing as aging of population progresses. Furthermore, in emerging countries, the needs for medical services are expanding because of economic and income growth. As a result, the annual growth rate of overseas market is expected to be 7 to 8%, which is much higher than that of the domestic market. Therefore, the Japanese companies in the industry are vigorously undertaking globalization of their businesses. In the global market, the global large companies such as Roche, Abbott, SIEMENS, and Beckman, whose sales are 200,000 to 900,000 million yen, are leading the market, and in order for Japanese companies to survive the competition, they must strengthen their competitiveness by, for example, developing unique products or systems. 1. What are Clinical Tests?
One type of clinical tests is the "Biological test" that directly examines the body using medical equipment such as X-ray, CT, MRI, electrocardiogram, and ultrasound. Another type of clinical tests is the "Laboratory test" that examines biological samples (specimens) obtained from people such as blood, urine/feces, and cells.The clinical test reagents made by EIKEN CHEMICAL are the ones used for medical laboratory tests. For example, they are used to test infectious diseases or to measure small amounts of blood contained in stool. They are made to support diagnosis. Most of these reagents are called in vitro diagnostics (IVD) and are regulated by the Pharmaceutical and Medical Device Act so reagent manufacturers file applications with PMDA (Pharmaceuticals and Medical Devices Agency) and obtain its approval. Users include hospitals, clinics, medical offices, medical test centers that carry out tests commissioned by medical institutions, health screening centers, public health centers, and institutions for health research, and others. 2. Major Products
EIKEN CHEMICAL mainly manufactures and sells the following types of reagents and medical devices.As they deal with a wide range of reagents, they not only sell their in-house products but also purchase and sell products from other companies. Major in-house products include fecal immunochemical test reagents, microbiological reagents, immunological and serological reagents, urinalysis test strips, genetic testing reagents, etc. The sales ratio of in-house products to other companies' products is approximately 60:40. The gross profit margin is approximately 55% for in-house products and approximately 35% for other companies' products. 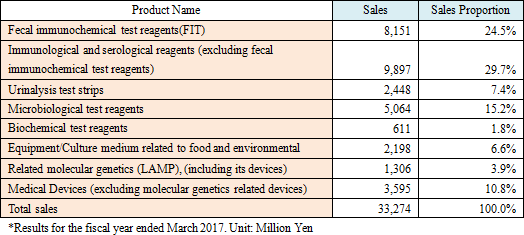 Fecal immunochemical test reagents
The major products for EIKEN CHEMICAL are reagents and sampling bottles for fecal immunochemical tests to specifically detect and measure human hemoglobin in feces as a colorectal cancer screening and diagnosis and are sold globally.
Immunological and serological reagents (excluding Fecal immunochemical test reagents)
Starting with reagents for "LZ Test 'EIKEN'," an automated analyzer, EIKEN CHEMICAL develops, manufactures, and sell a large selection of products such as reagents for tests of rheumatism and other autoimmune diseases (MMP-3, RF), inflammation markers (CRP, SAA) measurement reagents, reagents for tests of atrophic gastritis (Pepsinogen I and II), Helicobacter pylori antibodies in serum or plasma, and prostate-specific antigen (PSA). They also install and sell reagents for fully automated enzyme immunoassay devices and reagents for automatic glycohemoglobin analyzers from Tosoh Corporation. Urinalysis test strips
EIKEN CHEMICAL develops, manufactures, and sells "UROPAPER III 'EIKEN'," a urinalysis test strip for testing various items such as occult blood, protein and glucose, as well as the "UROPAPER Microbiological test reagents
Since its establishment, EIKEN CHEMICAL has been developing biological specimens as well as reagents for microbiological tests for food and environment in order to prevent infectious diseases and food poisoning. Currently, it develops, manufactures and sells various reagents that are effective for diagnosis and treatment of microorganism infection, such as mediums, powder mediums, antimicrobial susceptibility tests, and rapid test reagents.
Clinical chemistry test reagents
EIKEN CHEMICAL develops, manufactures and sells reagents for clinical chemistry tests including "EXDIA XL 'EIKEN'" series that assist to measure and analyze biological components in blood serum and urine, with a focus on the test items that are related to lifestyle related diseases.
Equipment/ Culture mediums related to food and environment
EIKEN CHEMICAL sells reagents for microbiological tests on food to detect food poisoning bacteria as well as reagents for environmental microbiological tests and equipment and devices to measure contamination of work environments.
Molecular genetics (LAMP)
In 1998, EIKEN CHEMICAL developed and patented an innovative gene amplification technology called "LAMP." The LAMP is "simple, rapid, and accurate" and is a critical tool for Eiken's future global expansion of its business. (Details are described below)
Medical devices
EIKEN CHEMICAL sells various types of automated analyzers. They contract manufacturing specialized equipment that uses their in-house reagent. Since beginning sales of "OC Sensor" in 1989, they have worked continuously on technological innovation and quality improvement of this fecal immunochemical test analyzer. Also, they offer the "US," an automated urine analysis device that uses Eiken's proprietary image processing system, the "BLEIA-1200," a fully automated biochemistry photogenetic immunoassay device that was the world's first of its kind in the clinical testing field, and "LoopampEXIA," a LAMP-based real time turbidity measuring device.
3. Sales structure
EIKEN CHEMICAL has 11 sales offices and 2 sales divisions in Japan. Its academic department supports sales promotion. Out of 694 employees (consolidated) during FY 2017, about 300 belong to the sales department. As for the sales channels for medical institutions such as hospitals, the Company's direct sales partners are medical wholesale companies, and it has businesses with almost all of the wholesale companies in the medical industry. For overseas sales, EIKEN CHEMICAL has basically 1 agency per country, and the sales and maintenance are commissioned to the agencies. EIKEN's products are exported to 47 countries (FY 2017). The high proportion of overseas sales is occupied by the sales in the USA, Italy, France, Spain, South Korea, and Taiwan. In addition to the Europe Branch in Amsterdam (the Netherlands), the Company is strengthening its manufacturing and sales structure through its consolidated subsidiary, "EIKEN CHINA CO., LTD.," as well as aiming to expand its businesses by setting a business office in China. In the future, it will explore the possibility of making the office as a local corporation, as the size expands. The overseas sales for FY 2017 are 4,086 million yen, out of which 2,414 million yen, 59.1%, is from the sales of fecal immunochemical test reagents. 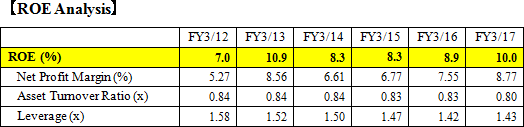 The Company aims to fortify priority measures, including developing high value-added products, generating new businesses and new markets, improving further profitability and productivity through reducing COGs rate and SG&A rate. Moreover, the Company plans to enhance overseas sales forces including increment of sakes staff especially, because it is needed to raise the in-house products rate from 60% to 70 or 80%. ROE for fiscal year March 2017 got to by 10% mainly due to the rise of profit rate as a result of some postponement of R&D investment. Pursuantly, ROE for fiscal year March 2018, is expected to be 7.0% (1) Products that Occupy High Share in the Market
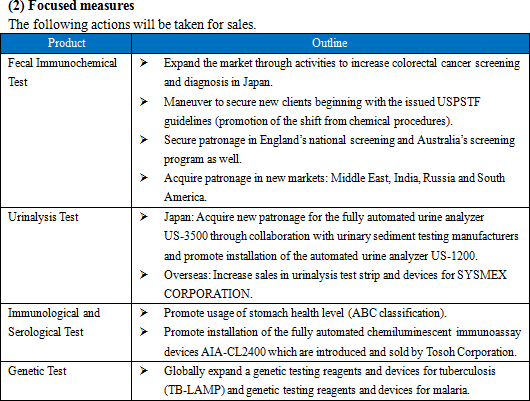
The share of Eiken's fecal immunochemical test reagents is ranked top (more than 60%) in the domestic market. Furthermore, many of their in-house products occupy high market share in the market, for example, urinalysis test strips occupying approximately 23% (ranked second) of the market, and microbiological reagents occupying approximately 18% (ranked second) of the market.The background to how Eiken's fecal immunochemical test reagents have come to hold such a high share of the market includes that in 1987, Eiken began sales of "OC-Hemodia," a visual determination method fecal immunochemical test reagents, a product that more closely conformed to user needs when compared to competitor's products, and that in 1989 they adopted the latex photometric immunoassay method and began sales of "OC-Sensor," the world's first fully automated analyzer. Also, the Health and Medical Service Act for the Aged was revised in 1992, making it possible to have fecal immunochemical test reagents as a method in colon cancer screening and diagnosis using public funds (no cost to the patient) which led to an accelerated spread and increased competition. But in 2001, Eiken began sales of the "OC-Sensor neo," with completely remodeled functions, which increased its market share. 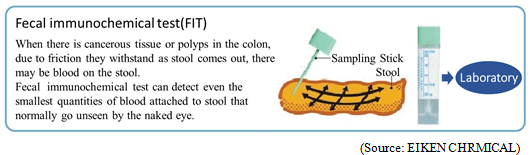 The immunochemical method used in Japan applies reagents that react only to human hemoglobin, and can process a large volume simultaneously. Meanwhile, in other countries, reagents for the chemical method (Guaiac method) based on old measuring principles are still used, which presents accuracy challenges. In 2011, the test guidelines in Europe have finally begun recommending automated analyzers that use the immunochemical method. As a result, the market is beginning to undergo a dramatic change. Furthermore, although the chemical method is also still common in the United States, which has the largest potential market, trends show a gradual shift toward the immunochemical method. Additionally, new guidelines on colorectal cancer screening by USPSTF (US Preventive Medicine Special Committee) was published in June 2016. These guidelines pointed that the immunization method is superior to the conventional chemical method and pursuantly, and assessed Eiken's fecal immunochemical test product, "the OC FIT-CHEK family of FITs" has the utmost inspection performance with high sensitivity and specificity. Besides, the large markets which are underdeveloping exist on the leading and emerging countries in Asia and Oceania, South America. Because the fecal immunochemical test market is a niche market, Japanese companies, the forerunners of the immunochemical method, own the most advanced technique, and hence Eiken's reagents and equipment are the global standard. (2) Focusing on research and development
EIKEN CHEMICAL is focusing on research and development of unique technologies as a research and development corporation, and the development of original products that respond to customers' needs, using the unique technologies. The number of staff assigned for research and development is about 100. The demand from the customers is higher quality of medicine. Specifically, they demand for higher differential diagnosis accuracy with high sensitivity and high quality and improved detection rate. In addition, easier usage will lead to reduction in the work of medical staff. Responding to such needs is critical. Since its establishment in 1939, EIKEN CHEMICAL has accumulated unique technologies for manufacturing reagents. Their unique technologies are applied to the measuring principles of their devices such as fecal occult blood test analyzer, automated urine analyzer, and biochemiluminescent immunoassay analyzer "BLEIA" that are designed to optimize the performance of the reagents. (3) Development of various types of products in various fields through alliance strategy
Because clinical test reagents have wide range of subjects and items, it is not possible for one company to develop, manufacture and sell all types of reagents. The other companies in the industry are focusing on the technologies and products that they are specialized in. However, as an integrated manufacturer of clinical test reagents, EIKEN CHEMICAL aims at stabilizing profit structure, expanding their own strengths through alliance strategy, and pursuing synergy effects such as complementing functions and acquiring new technologies, while dealing with a wide range of products and responding to the needs of customers and users such as medical institutions. Another reason why they cover various types of products in various fields is that they believe that covering wide range of clinical tests is their social responsibility to protect the health of the public, as is stated in their management philosophy: "protect the health of the public through health care services". (4) Competitive Advantages of the "LAMP"
Thus far the mainstream technology for amplifying genes as a process of gene tests has been what is called "PCR" Under such circumstances, in 1998, EIKEN CHEMICAL developed a unique technology called the "LAMP."Compared to the PCR, the "LAMP" offers the following superior characteristics and allows users to carry out simple, rapid and accurate gene tests.  EIKEN CHEMICAL is making focused efforts on infectious disease diagnostic test in order to establish the status of the LAMP. At the same time, it is promoting the use of the LAMP in other fields such as food production and processing, environment, agriculture/veterinary in order to spread and enhance recognition of the LAMP. In fact, the LAMP-based products have been commercialized one after another since 2002. Furthermore, for the same purposes, EIKEN CHEMICAL is actively giving licenses to external companies in order to build the LAMP camp. One of the major actions to spread the LAMP in the world is an alliance with "FIND." "FIND" stands for "Foundation for Innovative New Diagnostics" and is a non-profit organization recognized by the Swiss government, launched at a meeting of the United Nations World Health Assembly in May 2003. In its initial five years of existence, it received a grant from the Bill & Melinda Gates Foundation to start up their activities. Their goal is to develop and introduce affordable, simple and advanced diagnostic tests in order to eradicate infectious diseases in developing countries. FIND's scope of activities includes tuberculosis, malaria, and African sleeping disease. With tuberculosis, collaborative research between EIKEN CHEMICAL and FIND for a tuberculosis test using the LAMP began in July 2005. The purpose of this research is to improve the accuracy of tests by replacing the microscopy test (sputum smear test), which is the current practice in developing countries. As a result of this collaboration, improvements which are not possible with the conventional PCR such as simplified pretreatment (PURE), improved reagents storage (store at room temperature) and simplified devices have been made to enable the developing countries to carry out the procedure (TB-LAMP). This LAMP-based product was already launched in Japan in 2011. After that, in order to obtain endorsement from the WHO (World Health Organization), FIND has completed its clinical evaluation in 14 developing countries and submitted this information to the WHO. In consequence, the company has acquired the recommendation by WHO as an evaluation replaces with microscopic examination or as an inspection reinforcing microscopic examination in August 2016. According to a report on global tuberculosis announced by WHO on October 13, 2016, the number of patients suffering from tuberculosis in 202 countries all over the world in 2015 was 10.4 million, an increase of 0.8 million from 9.6 million in 2014. Additionaly, the number of deceases was 1.8 million, an increase of 0.3 million from 1.5 million in 2014. Most of them are inferred as matters of undiagnosed or untreated, and WHO indicates "the enforcement of countermeasures for the countries where access to diagnosis and treatment is not yet maintained is demanded". Following these situations, the company expects that dissemination and penetration of TB - LAMP contribute greatly to solve these problems. In addition to tuberculosis and other diseases listed above, EIKEN CHEMICAL and FIND also conduct collaborative research of reagents for leishmaniasis and Chagas disease. Also, EIKEN CHEMICAL is developing a next-generation compact fully automated genetic testing device and multi-item testing chip using the LAMP "Simprova". This equipment fully automates the process from specimen preprocessing (nucleic acid extraction and purification) to amplification and detection. By developing the unique protocol that exploits the LAMP's characteristics, the operation time that used to take over 2 hours with a conventional high purity nucleic acid extraction and purification device and an amplification and detection device combined, is now shortened to less than 30 minutes. First, a clinical performance test will be carried out with the goal of simultaneous detection of several respiratory infection causing germs but the usage application is extensive. It is anticipated that through these products, EIKEN CHEMICAL will accelerate the spread of the LAMP and establish its position as the global standard in a newly created market. ∗Gene amplification technology
Since the amount of genes found in a genetic test sample is extremely small, in order to detect genes, the targeted gene must be amplified first of all. Gene amplification technology, therefore, is crucially important for genetic testing.
∗African trypanosomiasis
An endemic found in tropical Africa, African trypanosomiasis is a serious tropical disease transmitted to HUMAN mbH by a protozoa called Trypanosoma brucei. The disease is transmitted by a tsetse fly. Trypanosoma in HUMAN mbH blood sucked by a tsetse fly develops and propagates inside the HUMAN mbH body in 2 to 5 weeks, before turning itself into a terminal Trypanosoma-type, which becomes a source of next round of infection. The disease causes fever, headache, and vomiting, and the patient falls into constant sleep. Since the patient cannot take meals, he or she becomes thin and complain of generalized weakness and, in many cases, leads to a complication and dies.
∗Leishmaniasis
Leishmaniasis is a disease transmitted by a protozoa called leishmania, and has various types such as visceral leishmaniasis (also known as black fever), Brazilian leishmaniasis that affects skin and mucous membranes, and tropical leishmaniasis which affects skin. All of these types are transmitted by blood-sucking insects, especially sandflies. Visceral leishmaniasis, after about three months incubation period, causes fever, sweating, diarrhea, etc. and, in about one month, causes a swollen liver and spleen, the patient develops an anemia and becomes weak if untreated, and may die in half a year to two years.
∗Chagas disease
Found in southern U.S. as well as Central and South America, Chagas disease is an infectious disease transmitted by Reduviidae, a kind of blood-sucking Triatominae. The disease does not develop symptoms immediately after infection; it usually has a latency period of about 30 years. It causes symptoms such as inflammation of sinews, liver and spleen, myalgia, myocarditis, cardiomegalia, encephalomyelitis, cardiac disturbance.
|
| Fiscal Year March 2017 Earnings Results |
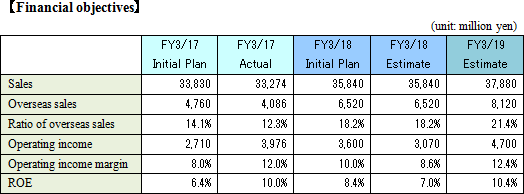
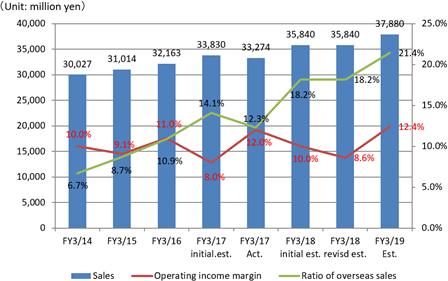
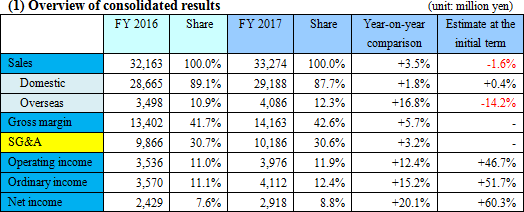 Sales increase from the previous term and double-digit rise in profit.
Sales were 33.2 billion yen, up by 3.5% compared to the previous year. Domestic sales saw fecal immunochemical test reagents, reagents for swift testing, and urinalysis test strip perform well. Overseas sales grew by double digits thanks to the increase in sales of urinalysis test strip and devices for SYSMEX CORPORATION although sales in North America and Europe for fecal immunochemical test reagents were sluggish. Operating income was 3.9 billion yen, up by 12.4% compared to the previous year. Gross profit increased due to the reduction in manufacturing costs in the company's own products, cost control was effective, and expected R&D expenses in the current term dragged onto the next period.Sales fell short of the estimate due to a slump in fecal immunochemical test reagents outside of Japan, yet profit greatly exceeded the estimate due to the deferment of R&D expenses. EIKEN CHEMICAL is aiming for a consolidated payout ratio of 30% or more; taking into consideration that profit exceeded the estimate, dividend at the end of the term increased from the originally anticipated 20 yen per share to 30 yen, with dividend for the entire year at 50 yen. The payout ratio will be 31.4%. 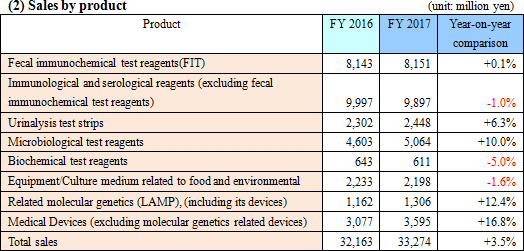 - Fecal immunochemical test reagents (FIT)
Sales were practically unchanged with only a 0.1% increase from the previous term.Domestic sales rose by 1.2% compared to the previous year. EIKEN CHEMICAL's efforts to promote installation of their Fully automated fecal occult blood analyzer - the OC Sensor PLEDIA - have led to the increase of new clients using it. In addition, the company has continued to focus on expanding the market through activities to heighten public awareness of screening for colorectal cancer. Meanwhile, overseas sales decreased by 2.3% compared to the previous year. Market deployment in the U.S. experienced a delay which required adjustments to inventory. French sales fell short of the estimate due to changes in laboratory procedure. In addition, the national health screening in England began belatedly. Fecal immunochemical test reagents are now covered by insurance in Germany and their use has been finalized for major testing centers. EIKEN CHEMICAL have been focusing on activities to secure new clients after receiving the U.S. Preventive Services Task Force's (USPSTF) newly-issued guidelines on screening for colon cancer (June 2016). The company has continued efforts to have their products used in national screenings for colorectal cancer in different countries and has managed to receive patronage in Qatar and Hanoi, Vietnam. - Immunological and serological reagents (excluding fecal immunochemical test reagents)
Sales decreased by 1.0% compared to the previous year.AIA-related Reagents introduced and sold by Tosoh Corporation saw sales drop due to fierce competition. LZ reagents primarily increased in the form of helicobacter pylori antibody test reagents. They conducted activities to increase usage of a stomach health evaluation (that uses a rating of A, B, or C) and promoted new patronage and sales of their interstitial pneumonia test reagent - KL-6. - Urinalysis test strips
Sales increased by 6.3% compared to the previous year.EIKEN CHEMICAL began selling US-1200, the successor to the automated urine analyzer US-1000, in August 2016. Moreover, new users of their fully automated urine analyzer US-3500 and their automated urine analyzer US-2200 increased. Clients using urinalysis test strip UROPAPER and UROPAPER α III 'EIKEN' grew as well. Outside Japan, the supply of urinalysis test strip to SYSMEX CORPORATION, which began in January 2016, contributed to the growth. - Microbiological reagents
Sales increased by 10.0% compared to the previous year.The sales of prepared media (Pore Media) fell due to the impact from changing to genetic screening in the form of fecal media testing. Meanwhile, selling "Imunocatch-regeonera" and "Imunocatch-pneumococcus" in sets met customer needs, leading to a major surge in sales of reagents for swift testing. Reagents for drug susceptibility testing saw growth in dry plates from securing new clients in complete packaged proposals conducted in unison with analyzers that identify the classification of microbes in the hospital market. - Related molecular genetics (LAMP)
Sales increased by 12.4% compared to the previous year.In Japan, reagents for LAMP products (tuberculosis complex and mycoplasma) grew and their bordetella pertussis detection reagent became covered by insurance in November 2016. Outside Japan, TB-LAMP acquired a recommendation from WHO in August 2016. Following this, the company signed a global sales contract with HUMAN mbH in Germany about TB-LAMP and malaria, excluding some regions, and has begun selling it. Patent revenue was 472 million yen, up by 6.5% from the previous term. - Medical devices
Sales increased by 16.8% compared to the previous year. Overseas medical equipment sales increased, such as urinalysis devices for SYSMEX CORPORATION.
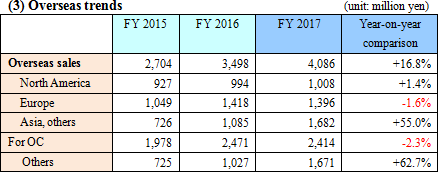 *North America
The slump in the consultation rate of the Veteran's Affairs' (VA) health care benefits program and the prolonged evaluation of switching to OC products left sales at a slight increase.
*Europe
The sales of fecal immunochemical test reagents (OC) to France and Spain fell short of the estimate due to changes in the screening program, resulting in a decline.
*Asia and Others
Urinalysis reagent and device sales to SYSMEX CORPORATION performed well.Sales channels for fecal immunochemical test reagents in the Middle East and Oceania expanded. *FIND business
A genetic testing procedure for tuberculosis (TB-LAMP) which utilizes the "Loopamp MTBC Detection Kit," "LoopampPURE DNA Extraction Kit," and "Loopamp LF-160, HomothermalEquipment with Fluorometer" via the LAMP, which was jointly developed with FIND, had its results recognized and received a recommendation from WHO.In the future, with the sales contract signed by HUMAN mbH in Germany, they will make way into the overseas market for tuberculosis and malaria genetic testing drugs and measuring devices (excluding China, South Korea, Taiwan and Thailand) with the LAMP. 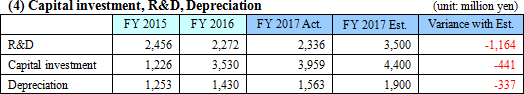 The depreciation method for buildings and structures was changed from a declining-balance to a straight-line. 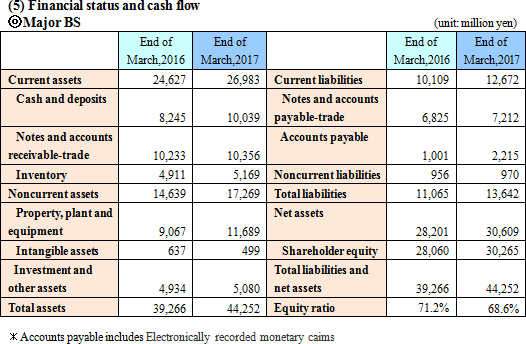 Current liabilities grew by 2,563 million yen due mainly to increases in unpaid amounts and accounts payable (trade and others). Noncurrent liabilities remained about the same and total liabilities rose by 2,577 million yen from the end of the previous term to 13,642 million yen. Net assets grew by 2,408 million yen from the end of the previous term to 30,609 million yen due mainly to an increase in retained earnings. As a result, the equity ratio decreased by 2.6% from 71.2% to 68.6% 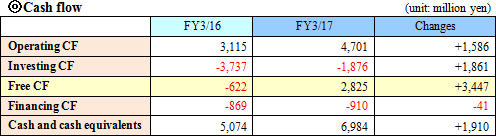 |
| Fiscal Year March 2018 Earnings Estimates |
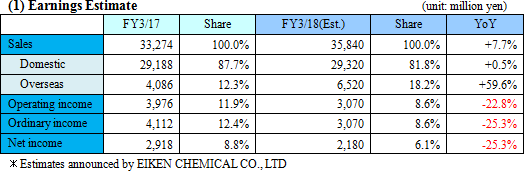 Despite sales increase, intensive R&D investment and others raise profit fall.
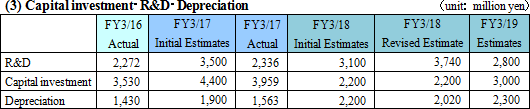
Sales are expected to increase by 7.7% from the previous term to 35.8 billion yen. Overseas sales, primarily in Europe where sales were short of the estimate last term, will be recovered.Operating income is anticipated to decrease by 22.8% compared to the previous year to 3 billion yen. EIKEN CHEMICAL has plans to increase SG&A expenses from sales costs for active sales promotions as well as augment traveling and packaging costs and boost IT investments to streamline work. They also intend to make intensive R&D investments which include the deferment of R&D expenses in the previous term. Dividend is expected to be equal to that in the previous term with 25 yen per share for interim and 25 yen per share for year-end, a total of 50 yen per share. The payout ratio is forecasted to be 42.0%.
   (4) LAMP Compact Fully Automated Genetic Testing System "Simprova"
EIKEN CHEMICAL has been developing a testing method and regents to make screening quicker and simpler than the traditional genetic testing method, and have succeeded in developing Simprova, a LAMP compact fully automated genetic testing system, which is highly sensitive and specific, able to quickly and easily test multiple items simultaneously. The Company is currently working to further develop the product for commodification.
◎ Characteristics
|
| Interview with the President Morifumi Wada |
|
"We have made good progress on cutting down costs, but it is still not enough. We will install IT system to increase the entire company's operational efficiency."
In the past few years, we have managed to make good progress on cutting down costs. However, this is due to mainly the ingenuity and efforts of factory workers, and partially the investment so far. I feel these management policies are still insufficient.I would like to thoroughly review the business operation of not only factory, but also the entire company, eliminate wasteful sequences and processes, and see if we can make things simpler. "To improve operational efficiency via complete optimization of the company" and "to revise the IT system of the entire company" which are outlined in mid-term managerial plan actually refers to simplifying things. In doing so, we will be able to further elevate quality and speed. It will be extremely important in order for us to become more formidable in our expansion overseas in the future. We will build up it within two or three years. 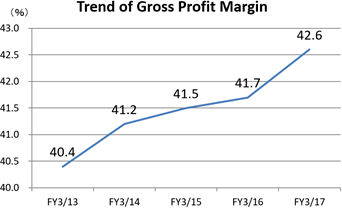 "Our lineup will be extended along with the expansion in the fecal immunochemical testing market, and we will further open up the overseas market."
Last term's overseas sales grew by double digits from the previous period, however they fell short of the estimate and were not completely satisfactory, with fecal immunochemical test reagent sales in North America and Europe experiencing a downswing.Each country had their own circumstances and in the short term, there can be difficulty in doing things with our company's efforts alone, however it is certainly true that the market continues to grow. Currently, our company operates in approximately 70 countries around the world. Awareness and other factors toward diagnosis greatly differs based on regulations, systems, and culture depending on the country, and so we need to use the correct approaches, however we have the advantage of highly precise immunization methods and being able to process a large quantity of it using our automated devices in the fecal immunochemical testing market. We will continue to make our mark in existing markets such as North America, Europe or other area as well as open new markets in the Middle East, Asia or others. In addition, most of the increase in overseas sales has primarily been in fecal immunochemical test products, however our lineup including genetic testing-related and immunological and serological test reagents have been extended, adding the surge in urinalysis reagents and devices supplied to SYSMEX Corporation in the previous term. I believe this will lead to overseas sales gaining more momentum in the future. TB-LAMP acquiring a recommendation by WHO proved how magnificent it is. We will make progress in sales primarily through an alliance with HUMAN mbH in Germany, however to do so, we will once again need to collect a large quantity of data in the countries to whom we intend to sell. Moreover, to have it employed in the developing countries whose funds are scarce, we will need to cooperate in funding with different foundations or any fund raisers and to receive the actual figures will take some more time. "We are concentrating our efforts on introducing a training program and developing our personnel in order to sufficiently draw out the potential in every single one of them."
We are working to revise our training system in regard to building HUMAN mbH assets which is the most important challenge for a company.As a labor shortage continues into the future, a training program that can draw out the potential in each individual will become more and more important. This is not only necessary for young workers, but also for executive officials who are in middle management and upper administration. I believe their proper roles and motivating them will be essential to the growth of the company. We will listen to feedback from outside agencies while working with the mindset of completely changing our personnel system for the entire company. |
| Conclusions |
|
|
| <Reference1: Mid-term Managerial Plan (FY 2017 to FY 2019)> |
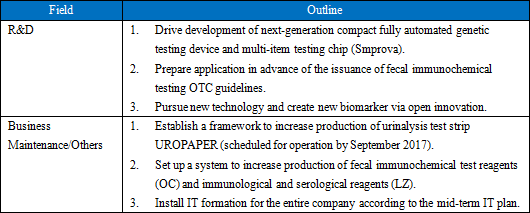
  Its growth strategies are (1) to expand the share of its products in the Japanese market, and (2) to accelerate global business expansion. As the investment for further growth, the company plans to (1) strengthen R&D and (2) develop a base for enhancing management efficiency. 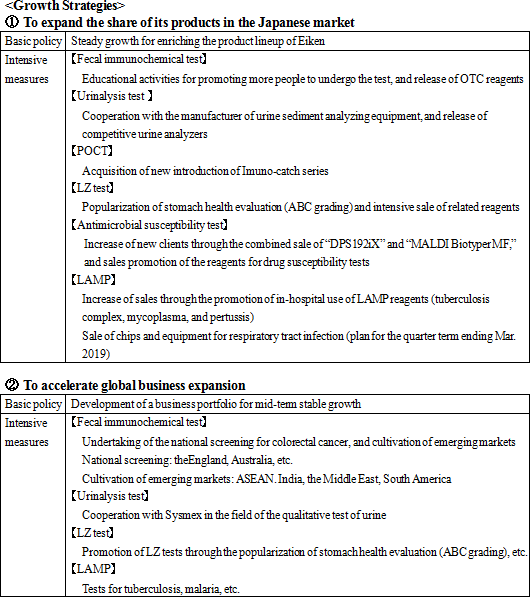 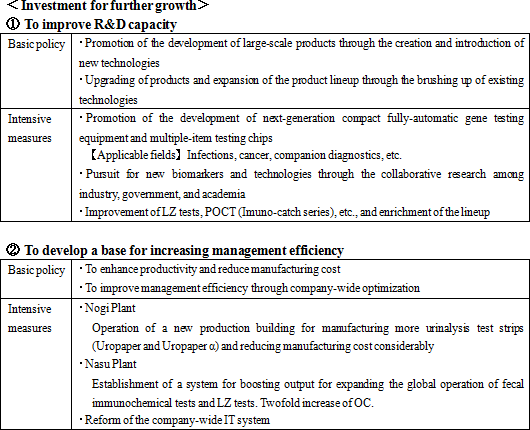 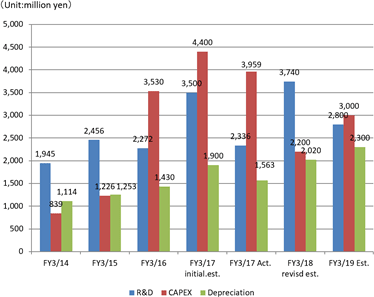 |
| <Reference2: Regarding Corporate Governance> |
 ◎ Corporate Governance Report
Last updated: submitted on June 23, 2017.<Reasons for Non-compliance with the Principles of the Corporate Governance Code (Excerpts)> The company has implemented every principle detailed in the Corporate Governance Code.  Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2017 Investment Bridge Co., Ltd. All Rights Reserved. |



