| EIKEN CHEMICAL (4549) |
|
||||||||
Company |
EIKEN CHEMICAL CO., LTD. |
||
Code No. |
4549 |
||
Exchange |
Tokyo Stock Exchange, First Section |
||
Industry |
Pharmaceuticals (manufacturing and sales) |
||
President |
Morifumi Wada |
||
HQ Address |
7 Yamaguchi building, 4-19-9 Taito, Taito-ku, Tokyo 110-8408, Japan |
||
Year-end |
End of March |
||
URL |
|||
*Share price is as of the end of May 27. The number of shares issued is from the latest financial settlement report (excluding treasury shares from the number of shares issued). ROE and BPS are based on actual results at the end of the previous term.
|
||||||||||||||||||||||||
|
|
|
| Key Points |
 |
| Company Overview |
|
It offers many products that occupy high market share including fecal immunochemical test reagents that occupy about 57% of the domestic share. Its unique gene amplification technology, "LAMP", is highly recognized in the world. With the fecal immunochemical test reagents, urinalysis test strips and LAMP, EIKEN is aiming to become a global corporation.
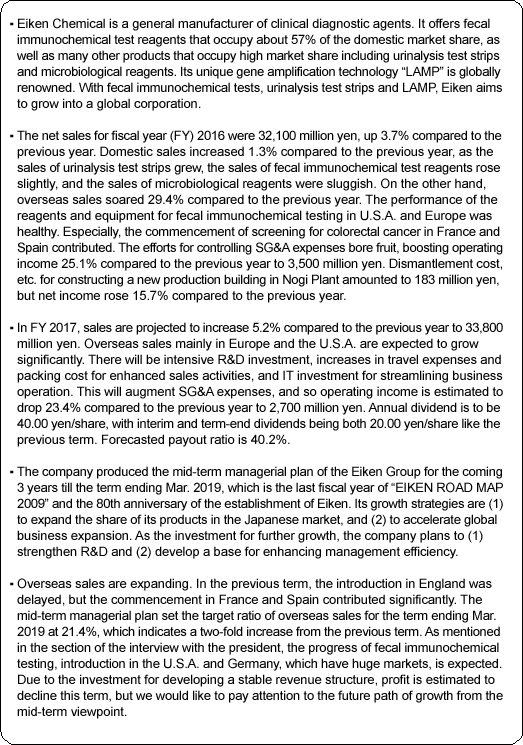
"Management Vision": EIKEN group is dedicated to leveraging expertise as a medical testing pioneer in order to increase corporate value by protecting the health of the public with products and services that customers can trust. "Motto": We EIKEN provide trustworthy quality, and develop with technology. EIKEN group formulates "EIKEN WAY" as its attitude toward each stakeholder, centering these philosophy, vision and motto. 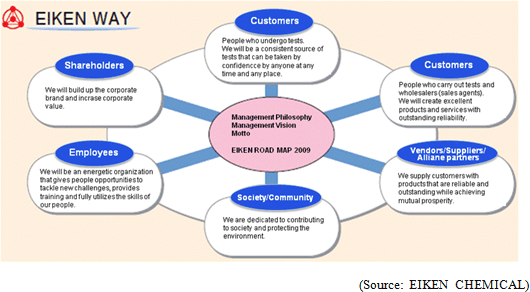 <Domestic Market>
The market scale of clinical reagents is about 338 billion yen and 557.3 billion yen (including research reagents and diagnostic devices) as of 2014 (survey by the Japan Association of Clinical Reagents Industries, or JACRI). In order to control rising medical costs, the Japanese government is focusing on preventive medicine such as special health check-ups (metabolic check-ups) and cancer screenings. It is expected that this, along with the aging population, will lead to an increase in the number of samples (number of specimens). Some negative factors include the impact of population decline as a result of decreasing birth rates and revision of medical treatment fees (reduction). However, the trends of laboratory test fees which had been subject to revision of insurance (medical laboratory test fees) show that, even though they were cut by some 40% from 1997 to 2006, the fees have been stable or only slightly reduced after 2007. (Laboratory test fee in fiscal 2016: -0.4%) This is a result of advocacy on the importance of preventive medicine and clinical diagnostics by the entire industry including Eiken. Thus, in the mid to long term, the domestic market is expected to slightly grow by 3% each year. According to the "Prospect of Clinical Laboratory Test Market, 2009" conducted by Yano Research Institute, Eiken Chemical ranked the 5th largest, occupying 5.8% market share, after Sysmex (6869, first section of Tokyo Stock Exchange), Roche Diagnostics (Japanese corporation of the Roche Group in Germany), Fujirebio (now known as Miraca Holdings. 4544, first section of Tokyo Stock Exchange), and Abbott Japan (Japanese corporation of the Abbott Group in the US). Out of the 126 member companies (as of April 2016) of JACRI mentioned above, about 80 are manufacturers, and there are about 15 companies with over 10 billion yen in sales. Most of them are small to medium sized companies. Because the test items of diagnostic tests range widely, each company has its own field of strength, and business segregation is already established in the industry. As a result, collaboration, such as supplying raw materials and products from other companies and manufacturing and selling them, is often observed. Against such a backdrop, the market is modestly growing. Therefore, there is currently no apparent trend of weeding out uncompetitive corporations. <Overseas Market>
The global clinical laboratory test reagent/device market is estimated to be US$ 56 billion and, by region, the market is occupied by the USA at 42%, followed by Europe at 33% and Asia/Pacific at 22%. The overseas market is over ten times larger than the domestic market. In developed countries, the number of tests is increasing as aging of population progresses. Furthermore, in emerging countries, the needs for medical services are expanding because of economic and income growth. As a result, the annual growth rate of overseas market is expected to be 7 to 8%, which is much higher than that of the domestic market. Therefore, the Japanese companies in the industry are vigorously undertaking globalization of their businesses. In the global market, the global large companies such as Roche, Abbott, SIEMENS, and Beckman, whose sales are 200,000 to 900,000 million yen, are leading the market, and in order for Japanese companies to survive the competition, they must strengthen their competitiveness by, for example, developing unique products or systems. 1. What are Clinical Tests?
One type of clinical tests is the "Biological test" that directly examines the body using medical equipment such as X-ray, CT, MRI, electrocardiogram, and ultrasound. Another type of clinical tests is the "Physiological function tests (laboratory tests)" that examines biological samples (specimens) obtained from people such as blood, urine/feces, and cells.The clinical test reagents made by Eiken Chemical are the ones used for medical laboratory tests. For example, they are used to test infectious diseases or to measure small amounts of blood contained in stool. They are made to support diagnosis. Most of these reagents are called in vitro diagnostics (IVD) and are regulated by the Pharmaceutical and Medical Device Act so reagent manufacturers file applications with PMDA (Pharmaceuticals and Medical Devices Agency) and obtain its approval. Users include hospitals, clinics, medical offices, medical test centers that carry out tests commissioned by medical institutions, health screening centers, public health centers, and institutions for health research, etc. 2. Major Products
Eiken Chemical mainly manufactures and sells the following types of reagents and medical devices.As they deal with a wide range of reagents, they not only sell their in-house products but also purchase and sell products from other companies. Major in-house products include fecal immunochemical test reagents, microbiological reagents, immunological and serological reagents, urinalysis test strips, genetic testing reagents, etc. The sales ratio of in-house products to other companies' products is approximately 60:40. The gross profit margin is approximately 55% for in-house products and approximately 35% for other companies' products. 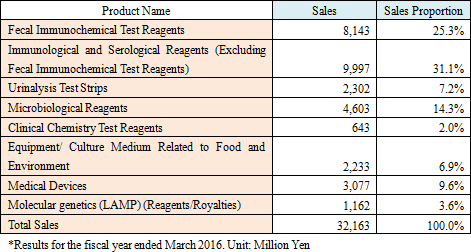 Fecal Immunochemical Test Reagents
The major products for Eiken Chemical are reagents and sampling bottles for fecal immunochemical tests to specifically detect and measure human hemoglobin in feces as a colorectal cancer screening and diagnosis and are sold globally.
Immunological and Serological Reagents (Excluding Fecal Immunochemical Test Reagents)
Starting with reagents for "LZ Test 'EIKEN'," an automated analyzer, Eiken Chemical develops, manufactures, and sell a large selection of products such as reagents for tests of rheumatism and other autoimmune diseases (MMP-3, RF), inflammation markers (CRP, SAA) measurement reagents, reagents for tests of atrophic gastritis (Pepsinogen I and II), Helicobacter pylori antibodies in serum or plasma, and prostate-specific antigen (PSA). vThey also install and sell reagents for fully automated enzyme immunoassay devices and reagents for automatic glycohemoglobin analyzers from Tosoh Corporation. Urinalysis Test Strips
Eiken Chemical develops, manufactures, and sells "UROPAPER III 'EIKEN'," a urinalysis test strip for testing various items such as occult blood, protein and glucose, as well as the "UROPAPER α III 'EIKEN'," a specialized test strip for fully automated urine analyzers.
Microbiological Reagents
Since its establishment, Eiken Chemical has been developing biological specimens as well as reagents for microbiological tests for food and environment in order to prevent infectious diseases and food poisoning. Currently, it develops, manufactures and sells various reagents that are effective for diagnosis and treatment of microorganism infection, such as mediums, powder mediums, antimicrobial susceptibility tests, and rapid test reagents.
Clinical Chemistry Test Reagents
Eiken Chemical develops, manufactures and sells reagents for clinical chemistry tests including "EXDIA XL 'EIKEN'" series that assist to measure and analyze biological components in blood serum and urine, with a focus on the test items that are related to lifestyle related diseases.
Equipment/ Culture Mediums Related to Food and Environment
Eiken Chemical sells reagents for microbiological tests on food to detect food poisoning bacteria as well as reagents for environmental microbiological tests and equipment and devices to measure contamination of work environments.
Medical Devices
Eiken Chemical sells various types of automated analyzers. They contract manufacturing specialized equipment that uses their in-house reagent. Since beginning sales of "OC Sensor" in 1989, they have worked continuously on technological innovation and quality improvement of this fecal immunochemical test analyzer. Also, they offer the "US," an automated urine analysis device that uses Eiken's proprietary image processing system, the "BLEIA-1200," a fully automated biochemistry photogenetic immunoassay device that was the world's first of its kind in the clinical testing field, and "LoopampEXIA," a LAMP-based real time turbidity measuring device.
Molecular genetics (LAMP)
In 1998, Eiken Chemical developed and patented an innovative gene amplification technology called "LAMP." The LAMP is "simple, rapid, and accurate" and is a critical tool for Eiken's future global expansion of its business. (Details are described below)
3. Sales structure
EIKEN CHEMICAL has 11 sales offices and 2 sales divisions in Japan. Its academic department supports sales promotion. Out of 637 employees (non-consolidated) during FY 2016, about 300 belong to the sales department. As for the sales channels for medical institutions such as hospitals, the Company's direct sales partners are medical wholesale companies, and it has businesses with almost all of the wholesale companies in the medical industry. For overseas sales, EIKEN CHEMICAL has basically 1 agency per country, and the sales and maintenance are commissioned to the agencies. EIKEN's products are exported to 43 countries (FY 2016). The high proportion of overseas sales is occupied by the sales in the USA, Italy, France, Spain, South Korea, and Taiwan. In addition to the Europe Branch in Amsterdam (the Netherlands), the Company is strengthening its manufacturing and sales structure through its consolidated subsidiary, "EIKEN CHINA CO., LTD.," as well as aiming to expand its businesses by setting a business office in China. In the future, it will explore the possibility of making the office as a local corporation, as the size expands. The overseas sales for FY 2015 are 3.498 billion yen, out of which 2.471 billion yen, 70.6%, is from the sales of fecal immunochemical test reagents and medical devices.  The Company aims to fortify priority measures, including developing high value-added products, generating new businesses and new markets, improving further profitability and productivity through reducing COGs rate and SG&A rate. Moreover, the Company plans to enhance overseas sales forces including increment of sakes staff especially, because it is needed to raise the in-house products rate from 60% to 70 or 80%. (The reason for the high ROE in FY 2013 was the increased net income margin from posting extraordinary income from land sales.) (1) Products that Occupy High Share in the Market
The share of Eiken's fecal immunochemical test reagents is ranked top (approximately 57%) in the domestic market. Furthermore, many of their in-house products occupy high market share in the market, for example, urinalysis test strips occupying approximately 23% (ranked second) of the market, and microbiological reagents occupying approximately 18% (ranked second) of the market.The background to how Eiken's fecal immunochemical test reagents have come to hold such a high share of the market includes that in 1987, Eiken began sales of "OC-Hemodia," a visual determination method fecal immunochemical test reagents, a product that more closely conformed to user needs when compared to competitor's products, and that in 1989 they adopted the latex photometric immunoassay method and began sales of "OC-Sensor," the world's first fully automated analyzer. Also, the Health and Medical Service Act for the Aged was revised in 1992, making it possible to have fecal immunochemical test reagents as a method in colon cancer screening and diagnosis using public funds (no cost to the patient) which led to an accelerated spread and increased competition. But in 2001, Eiken began sales of the "OC-Sensor neo," with completely remodeled functions, which increased its market share.  The immunochemical method used in Japan applies reagents that react only to human hemoglobin, and can process a large volume simultaneously. Meanwhile, in other countries, reagents for the chemical method (Guaiac method) based on old measuring principles are still used, which presents accuracy challenges. In 2011, the test guidelines in Europe have finally begun recommending automated analyzers that use the immunochemical method. As a result, the market is beginning to undergo a dramatic change. Furthermore, although the chemical method is also still common in the United States, which has the largest potential market, trends show a gradual shift toward the immunochemical method. This means that, in both developed and emerging countries in Europe, North America, Asia and Oceania, there is a large unexplored market. Because the fecal immunochemical test market is a niche market, Japanese companies, the forerunners of the immunochemical method, own the most advanced technique, and hence Eiken's reagents and equipment are the global standard. (2) Focusing on research and development
EIKEN CHEMICAL is focusing on research and development of unique technologies as a research and development corporation, and the development of original products that respond to customers' needs, using the unique technologies. The number of staff assigned for research and development is about 100. The demand from the customers is higher quality of medicine. Specifically, they demand for higher differential diagnosis accuracy with high sensitivity and high quality and improved detection rate. In addition, easier usage will lead to reduction in the work of medical staff. Responding to such needs is critical. Since its establishment in 1939, EIKEN CHEMICAL has accumulated unique technologies for manufacturing reagents. Their unique technologies are applied to the measuring principles of their devices such as fecal occult blood test analyzer, automated urine analyzer, and biochemiluminescent immunoassay analyzer "BLEIA" that are designed to optimize the performance of the reagents. (3) Development of various types of products in various fields through alliance strategy
Because clinical test reagents have wide range of subjects and items, it is not possible for one company to develop, manufacture and sell all types of reagents. The other companies in the industry are focusing on the technologies and products that they are specialized in. However, as an integrated manufacturer of clinical test reagents, EIKEN CHEMICAL aims at stabilizing profit structure, expanding their own strengths through alliance strategy, and pursuing synergy effects such as complementing functions and acquiring new technologies, while dealing with a wide range of products and responding to the needs of customers and users such as medical institutions. Another reason why they cover various types of products in various fields is that they believe that covering wide range of clinical tests is their social responsibility to protect the health of the public, as is stated in their management philosophy: "protect the health of the public through health care services". (4) Competitive Advantages of the "LAMP"
Thus far the mainstream technology for amplifying genes as a process of gene tests has been what is called "PCR" Under such circumstances, in 1998, Eiken Chemical developed a unique technology called the "LAMP."Compared to the PCR, the "LAMP" offers the following superior characteristics and allows users to carry out simple, rapid and accurate gene tests.  Eiken Chemical is making focused efforts on infectious disease diagnostic test in order to establish the status of the LAMP. At the same time, it is promoting the use of the LAMP in other fields such as food production and processing, environment, agriculture/veterinary in order to spread and enhance recognition of the LAMP. In fact, the LAMP-based products have been commercialized one after another since 2002. Furthermore, for the same purposes, Eiken Chemical is actively giving licenses to external companies in order to build the LAMP camp. One of the major actions to spread the LAMP in the world is an alliance with "FIND." "FIND" stands for "Foundation for Innovative New Diagnostics" and is a non-profit organization recognized by the Swiss government, launched at a meeting of the United Nations World Health Assembly in May 2003. In its initial five years of existence, it received a grant from the Bill & Melinda Gates Foundation to start up their activities. Their goal is to develop and introduce affordable, simple and advanced diagnostic tests in order to eradicate infectious diseases in developing countries. FIND's scope of activities includes tuberculosis, malaria, and African sleeping disease. With tuberculosis, collaborative research between Eiken Chemical and FIND for a tuberculosis test using the LAMP began in July 2005. The purpose of this research is to improve the accuracy of tests by replacing the microscopy test (sputum smear test), which is the current practice in developing countries. As a result of this collaboration, improvements which are not possible with the conventional PCR such as simplified pretreatment (PURE), improved reagents storage (store at room temperature) and simplified devices have been made to enable the developing countries to carry out the procedure. This LAMP-based product was already launched in Japan in 2011. Presently, in order to obtain endorsement from the WHO (World Health Organization), FIND has completed clinical evaluation in 14 developing countries and submitted this information to the WHO. The company is estimated to receive a recommendation soon. In addition to tuberculosis and other diseases listed above, Eiken Chemical and FIND also conduct collaborative research of reagents for leishmaniasis and Chagas disease. Also, Eiken Chemical is developing a next-generation compact fully automated genetic testing device and multi-item testing chip using the LAMP. This equipment fully automates the process from specimen preprocessing (nucleic acid extraction and purification) to amplification and detection. By developing the unique protocol that exploits the LAMP's characteristics, the operation time that used to take over 2 hours with a conventional high purity nucleic acid extraction and purification device and an amplification and detection device combined, is now shortened to less than 30 minutes. First, a clinical performance test will be carried out with the goal of simultaneous detection of several respiratory infection causing germs but the usage application is extensive. It is anticipated that through these products, Eiken Chemical will accelerate the spread of the LAMP and establish its position as the global standard in a newly created market. ∗Gene amplification technology
Since the amount of genes found in a genetic test sample is extremely small, in order to detect genes, the targeted gene must be amplified first of all. Gene amplification technology, therefore, is crucially important for genetic testing.
∗African trypanosomiasis
An endemic found in tropical Africa, African trypanosomiasis is a serious tropical disease transmitted to humans by a protozoa called Trypanosoma brucei. The disease is transmitted by a tsetse fly. Trypanosoma in human blood sucked by a tsetse fly develops and propagates inside the human body in 2 to 5 weeks, before turning itself into a terminal Trypanosoma-type, which becomes a source of next round of infection. The disease causes fever, headache, and vomiting, and the patient falls into constant sleep. Since the patient cannot take meals, he or she becomes thin and complain of generalized weakness and, in many cases, leads to a complication and dies.
∗Leishmaniasis
Leishmaniasis is a disease transmitted by a protozoa called leishmania, and has various types such as visceral leishmaniasis (also known as black fever), Brazilian leishmaniasis that affects skin and mucous membranes, and tropical leishmaniasis which affects skin. All of these types are transmitted by blood-sucking insects, especially sandflies. Visceral leishmaniasis, after about three months incubation period, causes fever, sweating, diarrhea, etc. and, in about one month, causes a swollen liver and spleen, the patient develops an anemia and becomes weak if untreated, and may die in half a year to two years.
∗Chagas disease
Found in southern U.S. as well as Central and South America, Chagas disease is an infectious disease transmitted by Reduviidae, a kind of blood-sucking Triatominae. The disease does not develop symptoms immediately after infection; it usually has a latency period of about 30 years. It causes symptoms such as inflammation of sinews, liver and spleen, myalgia, myocarditis, cardiomegalia, encephalomyelitis, cardiac disturbance, etc.
|
| Fiscal Year March 2016 Earnings Results |
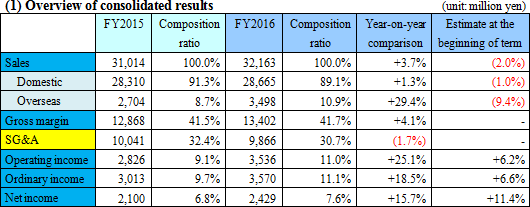 Sales and profit grew slightly. Overseas sales were favorable, while domestic sales remained on a plateau.
Sales were 32,100 million yen, up 3.7% from the previous term. Domestic sales increased 1.3% compared to the previous year, as the sales of urinalysis test strips, etc. grew, the sales of fecal immunochemical test reagents rose slightly, and the sales of microbiological reagents were sluggish. On the other hand, overseas sales soared 29.4% compared to the previous year. The performance of the reagents and equipment for fecal immunochemical testing in U.S.A. and Europe was healthy. Especially, the commencement of screening for colorectal cancer in France and Spain (Madrid and Barcelona) contributed. The efforts for controlling SG&A expenses bore fruit, boosting operating income 25.1% compared to the previous year to 3,500 million yen. A foreign exchange gain in the previous term turned into a foreign exchange loss, but the ordinary income increased 18.5% compared to the previous year. Dismantlement cost, etc. for constructing a new production building in Nogi Plant amounted to 183 million yen, but net income rose 15.7% compared to the previous year. 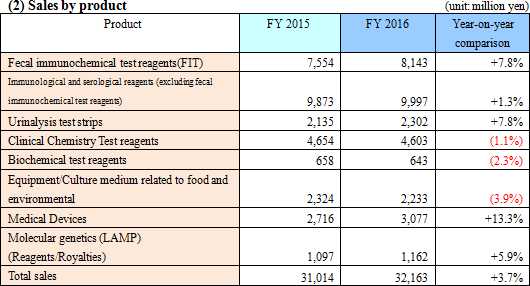 - Fecal immunochemical test reagents (FIT)
Sales increased 7.8% compared to the previous year, but the growth rate of domestic sales was as low as 1.7%. The company kept striving to increase the market by popularizing the screening for colorectal cancer. In addition, the company made efforts to increase the sales of fecal immunochemical test reagents, by promoting the installation of "OC Sensor PLEDIA," which is fecal occult blood analyzer released in Nov. 2014. On the other hand, overseas sales rose 24.9% compared to the previous year, through the sales promotion of reagents inU.S.A., the introduction of the screening for colorectal cancer in France and Spain (Madrid and Barcelona), the expansion of sales in Australia and New Zealand, etc. However, the introduction in England was delayed, and so sales did not reach the initial target. - Immunological and serological reagents (excluding fecal immunochemical test reagents)
Sales increased 1.3% compared to the previous year. The performance of "AIA-related reagents," which are procured from Tosoh Corporation and sold, was at the same level as that in the previous year. The evaluation of stomach health level (ABC grading) was promoted, and then the sales of the LZ test for the antibody test of Helicobacter pylori increased three times from the previous term. In Feb. 2016, the company released the new reagent LZ Test KL-6.
- Urinalysis test strips
Sales grew 7.8% compared to the previous year. The fully automatic urine analyzer "US-3500," which was released in Feb. 2015, was installed at more institutions, and the sales of the test paper "Uropaper α III Eiken" increased. Outside Japan, the sales of the urinalysis test strips targeted at Italy rose, and the company started supplying urinalysis test strips, etc. to Sysmex Corporation in Jan. 2016. In addition, the company commenced the construction of a new production building in Nov. 2015, and started preparing for the installation of new equipment for urine test paper. - Microbiological reagents
Sales dropped 1.1% compared to the previous year. The sales of prepared media (Pore Media) declined, and the performance of reagents for swift testing, mainly Imunocatch-Noro, slowed down. As the lineup of products for respiratory tract infections, the company released "Imunocatch-RSV" in Aug. 2015 and "Imunocatch-pneumococcus" in Jan. 2016.The sales of the reagents for drug susceptibility testing increased. - Clinical chemistry test reagents
Sales dropped 2.3% compared to the previous year.
- Equipment /Culture medium related to food and environment
Sales declined 3.9% compared to the previous year.
- Medical Devices
Sales increased 13.3% compared to the previous year, because the results of OC Sensor PLEDIA inside and outside Japan and US-3500 in Japan were healthy.
- Molecular genetics(LAMP)
Sales grew 5.9% compared to the previous year. The sales of the reagents for LAMP products (tuberculosis complex, malaria, and mycoplasma) increased. The development of next-generation compact fully-automatic genetic testing equipment and chips for testing multiple items progressed.Outside Japan, the company continuously promoted activities for obtaining a recommendation from WHO for tuberculosis testing. The company is preparing distributorship contracts for the global expansion of tuberculosis and malaria testing, and the tuberculosis testing was adopted in the private technology distribution project of JICA in the Philippines. The income from patent royalties dropped slightly year on year to 471 million yen. 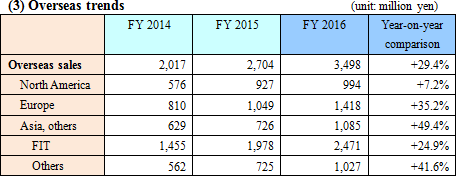 *North America
The company promoted the sales of the reagents and equipment for fecal immunochemical testing.
*Europe
The screening for colorectal cancer began in France and Spain (Madrid and Barcelona). On the other hand, in England, the commencement of national screening is delayed.
*Asia, etc
The company concentrated on the introduction and sales promotion of fecal immunochemical test reagents in Australia, New Zealand, etc., made efforts to expand sales channels in ASEAN, India, etc.; and promoted the sales of LAMP products, the reagents and analyzer for fecal immunochemical testing in China.
*FIND business
WHO is analyzing data, to determine whether or not to give a recommendation for tuberculosis testing. It is still to be determined, but the company considers that it is at the final stage.
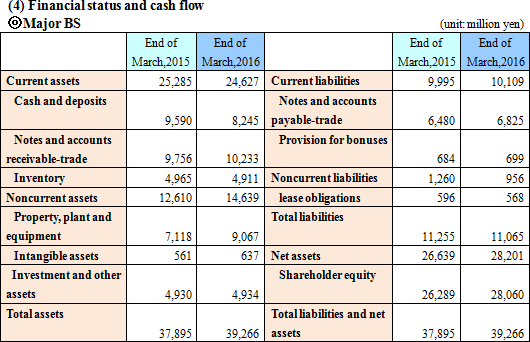 Current liabilities augmented 114 million yen from the end of the previous term, due to the increase in notes and accounts payable-trade, while noncurrent liabilities dropped 304 million yen. Consequently, total liabilities decreased 190 million yen.Net assets increased 1,562 million yen from the end of the previous term, due to the growth of retained earnings, etc. As a result, equity ratio rose 1.4% from 69.8% to 71.2%. 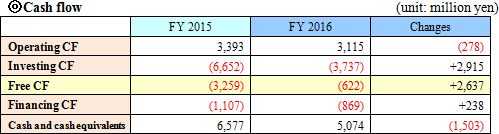 In the current term, long-term debts were not repaid, and so financing CF recovered slightly. The cash position declined. |
| Fiscal Year March 2017 Earnings Estimates |
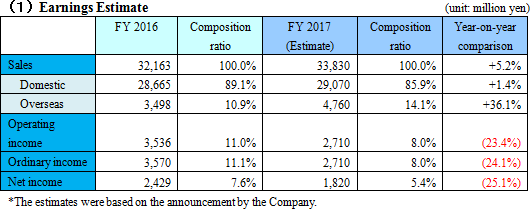 Sales will grow and profit will drop, due to the cultivation of overseas markets, intensive R&D investment, etc.
Sales are projected to increase 5.2% compared to the previous year to 33,800 million yen. Overseas sales mainly in Europe will grow considerably this term, too.Operating income is estimated to decrease 23.4% compared to the previous year to 2,700 million yen, due to the intensive R&D investment, increases in travel expenses and packing expenses for enhanced sales activities, IT investment for increasing efficiency, etc. As for dividends, interim, term-end, and annual dividends are estimated to be 20.00 yen/share, 20.00 yen/share, and 40.00 yen/share, respectively. Forecasted payout ratio is 40.2%. |
| Regarding the Mid-term Managerial Plan (FY 2017 to FY 2019) |
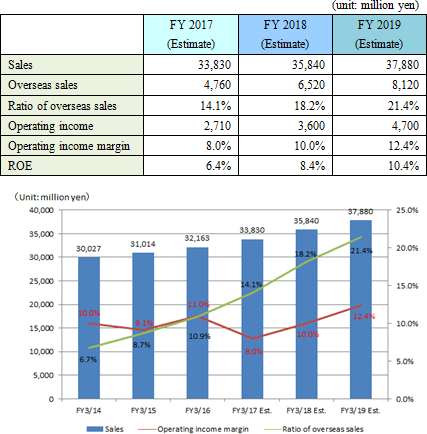 Its growth strategies are (1) to expand the share of its products in the Japanese market, and (2) to accelerate global business expansion. As the investment for further growth, the company plans to (1) strengthen R&D and (2) develop a base for enhancing management efficiency. 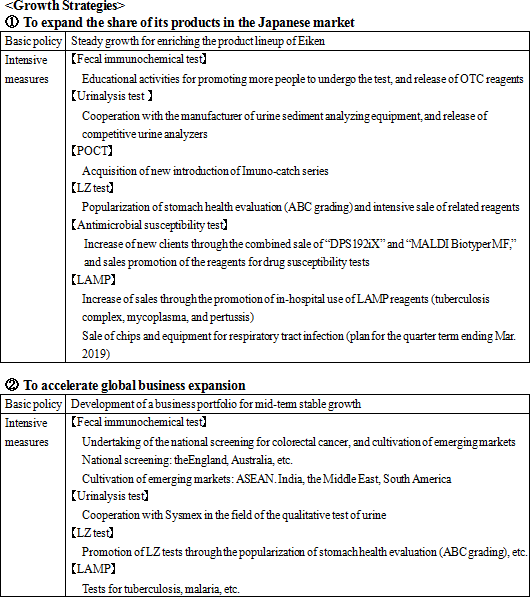 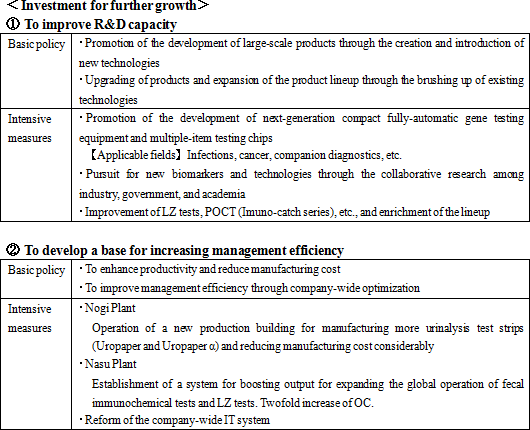 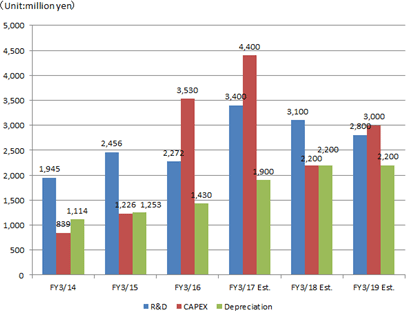 |
| Interview with President Morifumi Wada |
|
"Inside and outside Japan, there is room for the expansion of the market for fecal immunochemical test reagents. We will start redeveloping a base for cultivating overseas markets."
"Under the corporate culture in which freewheeling ideas are emphasized, core technologies will be brushed up further. We will also try to create new business and markets."
"We concentrate on the development of a base for becoming an enterprise that can earn profit stably."
|
| Conclusions |
|
Due to the investment for developing a stable revenue structure, profit is estimated to decline this term, but we would like to pay attention to the future path of growth from the mid-term viewpoint. |
| Reference: New Management Framework "EIKEN WAY" and "EIKEN ROAD MAP 2009" |
|
EIKEN CHEMICAL set a goal for 2018, the company's 80th anniversary year, and established "EIKEN WAY" and "EIKEN ROAD MAP 2009" in March 2009, as a fundamental policy to promote "winning management."
Furthermore, securing safety and compliance with laws and ordinances are becoming increasingly important management challenges. As a result, the gap between corporations is expected to be larger. Under these circumstances, in order to achieve steady growth and sustainable enhancement of corporate values, EIKEN CHEMICAL recognizes that it is essential to clarify the goal of EIKEN group, optimize the efficiency of management resources, and implement the strategies to utilize environmental changes with new visions more quickly and boldly. Based on this recognition, it established the "EIKEN WAY" as a plan to practice solid management and the "EIKEN ROADMAP 2009" as basic principles to promote "winning management" with long term goals. 1. Business domain
EIKEN CHEMICAL set the "clinical diagnostics business" and "food and environment testing business" among healthcare businesses as their main business domains for which they can utilize the technologies and strengths owned by the EIKEN Group to achieve steady growth and increase in profitability. Furthermore, in these domains, they are aiming at creating new businesses for future growth.
2. EIKEN ROAD MAP 2009 Grand Vision
We will transform EIKEN into a global corporation by 2018 that can leverage expertise as a medical testing pioneer to protect the health of the public.
3. EIKEN ROAD MAP 2009 Principles of Action
 4. Fundamental policy
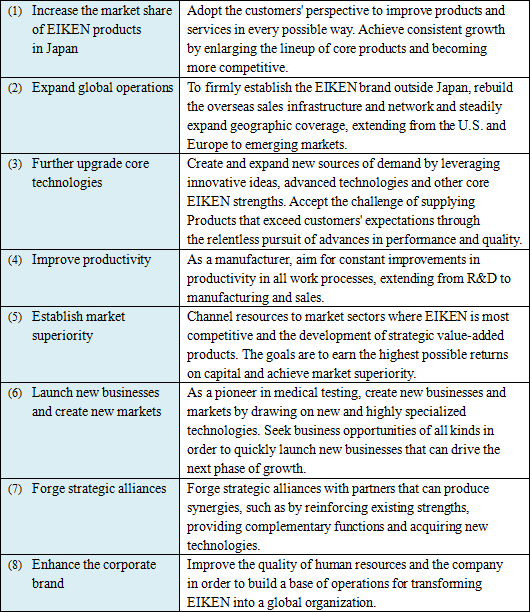 5. Management goal
To become one of the global medical testing corporations in the future, we will strive to steadily enhance profitability while creating solid business foundation. Our goal for the time being is to maintain over 10% of overseas sales ratio and over 10% of consolidated sales profit margin.
Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2016 Investment Bridge Co., Ltd. All Rights Reserved. |



