| Linical Co., Ltd. (2183) |
|
||||||||
Company |
Linical Co., Ltd. |
||
Code No. |
2183 |
||
Exchange |
First Section, TSE |
||
Industry |
Service |
||
CEO |
Kazuhiro Hatano |
||
HQ Address |
10 Fl., Shin-Osaka Brick Building, 6-1 Miyahara 1-chome, Yodogawa-ku, Osaka, Japan |
||
Year-end |
March |
||
URL |
|||
* Stock price as of closing on December 4, 2015. Number of shares issued at the end of the most recent quarter excluding treasury shares.
* ROE uses the result from the previous year end. BPS derives from the result of the second quarter for fiscal year March 2016. EPS uses an estimate at the beginning of this term. |
||||||||||||||||||||||||
|
|
* Estimates are those of the Company. An additional dividend of ¥2.5 per share was paid to commemorate the move of the Company's shares to the First Section of the Tokyo Stock Exchange during FY3/13
* A 2 for 1 stock split is expected to be performed on January 1, 2016. While dividend payment has been reduced to ¥7 per share, the actual dividend payment remains unchanged at ¥14 per share after the stock split is considered. We present this Bridge Report about Linical Co., Ltd. and details of first half of fiscal year March 2016 earnings. |
|
| Key Points |
 |
| Company Overview |
|
In the CRO business, Linical specializes in the most critical stages of clinical trials of phase II and III studies including major activities such as monitoring. Furthermore, Linical is able to differentiate itself from its competitors by focusing upon schizophrenia, depression, Alzheimer's disease and other central nervous system (CNS) diseases, oncology, and other highly difficult disease realms. (Compared with these disease realms, it is more difficult to differentiate services in diseases realms associated with adult lifestyle habits where competition is intense.) At the same time, Linical focuses upon specific patient realms in the contract medical affairs (CMA) business and leverage its knowhow established in the CRO business to provide product marketing and post market launch data planning and collection tasks to differentiate its CSO business from that of its competitors, who primarily provide the service of medical representative dispatch. Linical's main customers include Takeda Pharmaceutical Company Limited, Shionogi & Company, Limited, Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd. and other major pharmaceutical companies. Phase II clinical trials are conducted to test the safety, efficacy, usage, and dosage of pharmaceutical products. Phase III clinical trials take these results and confirm them in actual treatment conditions to test for efficacy and safety. <Corporate History>
Linical Co., Ltd. was established in June 2005 by nine members who worked at Fujisawa Pharmaceutical Co., Ltd. (Currently known as Astellas Pharma Inc.) on the development of immunosuppressant drugs. Established with the objective of becoming an ideal drug development outsourcing (CRO) company from Osaka, Linical focused its efforts upon the realms of central nervous system diseases (CNS) and oncology since its founding, and received one of its first orders from Otsuka Pharmaceutical Company shortly after its establishment. Thereafter, the Company fortified its staffing as part of its efforts to strengthen its order taking capabilities. In addition, Linical is benefitting from the bountiful experiences of its employees, who had worked at foreign pharmaceutical companies in the realm of oncology pharmaceutical product development. Consequently, Linical is successfully expanding orders in the near term.With its advance into the site management organization (SMO, clinical trial facility support organization) business, Aurora Ltd. was turned into a subsidiary in January 2006. However, all shares held in Aurora were sold in May 2007 in order to focus management resources upon the CRO business. In July 2008, Linical USA, Inc. was established in California, the United States to provide support to Japanese pharmaceutical companies seeking to enter the United States market. Also in October of the same year, Linical listed its shares on the Mothers Market of the Tokyo Stock Exchange, and subsequently moved its listing to the First Section of the Tokyo Stock Exchange in March 2013. In May 2013, wholly-owned subsidiaries, Linical Taiwan Co., Ltd. and Linical Korea Co., Ltd. were established in Taiwan and Korea, respectively. In April 2014, Linical teamed up with its Linical Korea to acquire the Korean CRO company P-pro. Korea Co., Ltd. In October 29, 2014, all of the shares of Nuvisan CDD Holding GmbH, which conducts CRO business in Europe, were acquired and it was converted to a 100% owned subsidiary effective on December 1, 2014. In order to strengthen the collaboration within the Group, the company name of Nuvisan CDD was changed to Linical Europe GmbH. 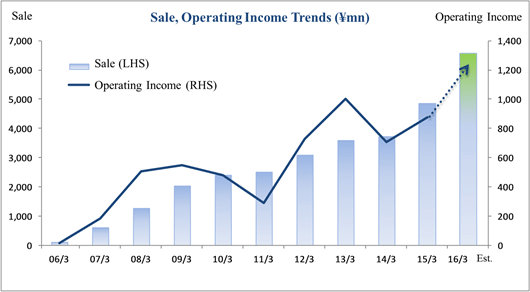 <Business Description>
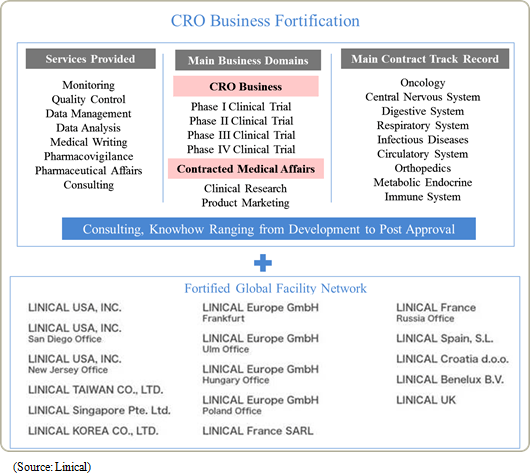
Linical's business can be divided into the two main business segments of the CRO and contract medical affairs (CMA), with each accounting for 92.5% and 7.5% of fiscal year March 2015 sales, respectively. In the CRO business, the Company focuses upon contract clinical trial operations for the development of pharmaceutical products by pharmaceutical companies. Furthermore, the CRO business specializes in "monitoring services," which includes the "quality control" and "consulting" services. At the same time, the CMA business specializes in the provision of contract support services for clinical research and product marketing as a means of differentiating itself from its competitors, which primarily focus upon medical representative (MR) dispatch.(Note) The previous segment category "CSO business" was changed to "contract medical affairs business" from the first quarter of fiscal year March 2016 CRO Business
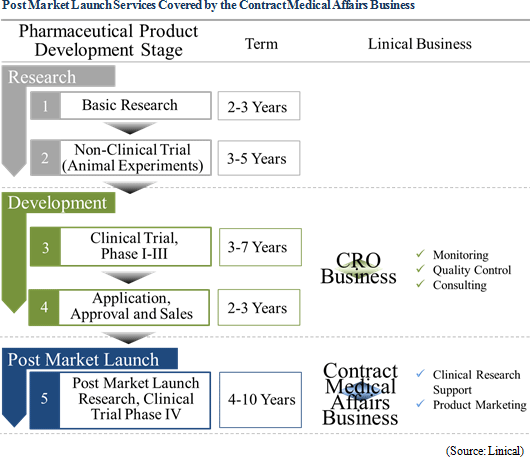
In the main CRO business, Linical seeks to provide high quality and highly effective clinical trial support that contributes to the quick market launch of new drugs, and boasts of staff with high skill levels and bountiful experiences. Also, facilities have been established in Asia (Korea, Taiwan, Singapore and other countries), Europe, and the United States in order to respond to the expanding needs for global studies. Linical provides comprehensive one-stop shopping responses ranging from pharmaceutical affairs planning, implementation plan creation, monitoring, data management, data analysis, and pharmarcovigilance. With regards to globally conducted joint trials, Linical's Osaka headquarter assumes the contact function and is staffed with employees who are well versed in pharmaceutical development information in various countries. The Company also boasts of the ability to provide a development environment that allows for internationally conducted joint testing to be conducted flexibly in the Japanese language. Amongst new drug development projects that require between 10 to 20 years, Linical focuses upon the "phase II and III clinical trials", which are the highly important parts of the overall clinical trial process and typically require between three to seven years, and it provides "monitoring", "quality control", and "consulting" services. Furthermore, support services designed to steadfastly and quickly realize new drug development, including highly reliable data collection services, are provided. Furthermore, Linical seeks to respond to the needs of client pharmaceutical companies in the highly difficult realms of oncology and central nervous system disorders.
 Contract Medical Affairs Business
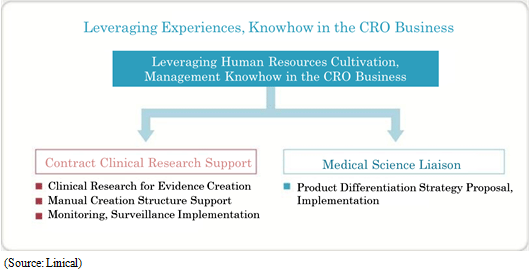
Clinical research after pharmaceutical product approval entails evidence based medicine (EBM) data creation related to activities designed to improve the quality of medical treatment including validation of effectiveness, confirmation of safety, interaction consideration for pharmaceutical product usage in the clinical work front. In recent years, facilitation of rules for clinical research has progressed, and the amount of corporate led clinical research is on the rise, in addition to the traditional clinical research led by physicians. Linical has been able to implement clinical research that responds to the most recent information in global applications not only in intervention studies, but also in contract observational studies, database research and other various clinical research by leveraging its knowhow cultivated in the realm of clinical trials. In the future, medical affairs divisions (MA) and medical science liaisons (MSL) will lead the way to gather opinions from key opinion leaders (KOL) in various disease realms in line with the enhancement of increasing fairness and transparency of marketing activities of pharmaceutical companies. Various tasks are conducted within the MSL division including key opinion leader engagement, advice board operations, and lecture operations, making its role progressively more important. Linical will leverage its experiences in operating manuals creation, regional opinion leader support, clinical research procedure manual creation support and consulting services to provide medical science liaison support on a global basis.
 New Drug Discovery Support Business
Linical provides a wide range of services that respond to the needs of pharmaceutical product development tasks including contract clinical trial monitoring, new drug marketing support, development plan proposal, and clinical trial plan creation that fully satisfy the requirements of client pharmaceutical companies in the new drug discovery support business. Moreover, Linical proposes new drug discovery solutions that reduce the risk of development by leveraging funds and subsidies to develop compounds that become the "seeds of medicine" in the initial stages of pharmaceutical product development process.
 <Strengths>
(1) Concentrated Specialized Knowledge, Knowhow, Experience that Respond to Pharmaceutical Companies' Needs
In general, a long period of time of between 10 to 18 years is required to obtain approval for the commercial launch of new drugs into the market. In this overall process, clinical trials require between three to seven years to complete, and lack of preparation, data, and other issues can lead to delays in the progress of trials and in the market launch of new drugs. Linical leverages its bountiful experience in the CRA services, including its highly accurate and speedy data gathering capability, other knowhow to predict and prevent various problems from occurring and ability to ensure that the "clinical trials progress smoothly," allowing it to provide accurate explanations of the overall clinical development process at the orientation stage to clients. Furthermore, a characteristic of Linical's specialized CRO service is its focus upon the three highly management-efficient tasks mentioned below.
① Linical specializes in the three core tasks ("monitoring", "quality control" and "consulting") of development, and it maintains a structure that is able to perform 100% of this contract work internally.
② The Company focuses upon the two most important stages of clinical trials of phase II and III.
③ Linical focuses upon major pharmaceutical companies that possess bountiful pharmaceutical product development information.
(2) Strengths in CNS, Oncology Monitoring, Highly Difficult Realms with Fewer Competitors
One of Linical's strengths is its specialization in monitoring services for highly difficult disease realms of the central nervous system and oncology, where there are fewer competitors. For example, in the realm of oncology evaluating symptoms to determine whether they are the result of side effects of drugs or the cancer itself is very difficult. And in the realm of central nervous system diseases, evaluating the efficacy of drugs prescribed for patients afflicted with Alzheimer's disease is also highly difficult. Therefore, sophisticated responses and expertise in monitoring are necessary in these difficult realms. In addition, new drug development for patients of acute and intractable diseases (Difficult diseases), which are also highly difficult disease realms, oncology and CNS disease realms is very active (There are only a few CROs that can respond in these realms). Evaluation of the efficacy of drugs for adult lifestyle-related diseases is far easier because conditions of patients undergoing clinical trials tend to be relatively stable (For example, data gathering of blood sugar levels in diabetes patients).New drug development trends are shifting from adult lifestyle related-diseases towards the realms of oncology and CNS, where treatment satisfaction is low. However as stated above, the safety evaluation of oncology drugs and the efficacy evaluation of CNS drugs are more difficult. Therefore, there is a trend of aggregation of contracts to CROs with bountiful track records in these realms. Order backlog in the realms of oncology, central nervous system, acute diseases and other intractable diseases are expanding. 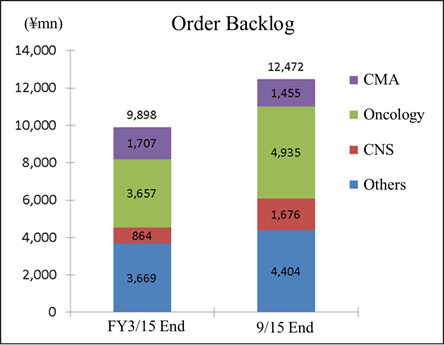 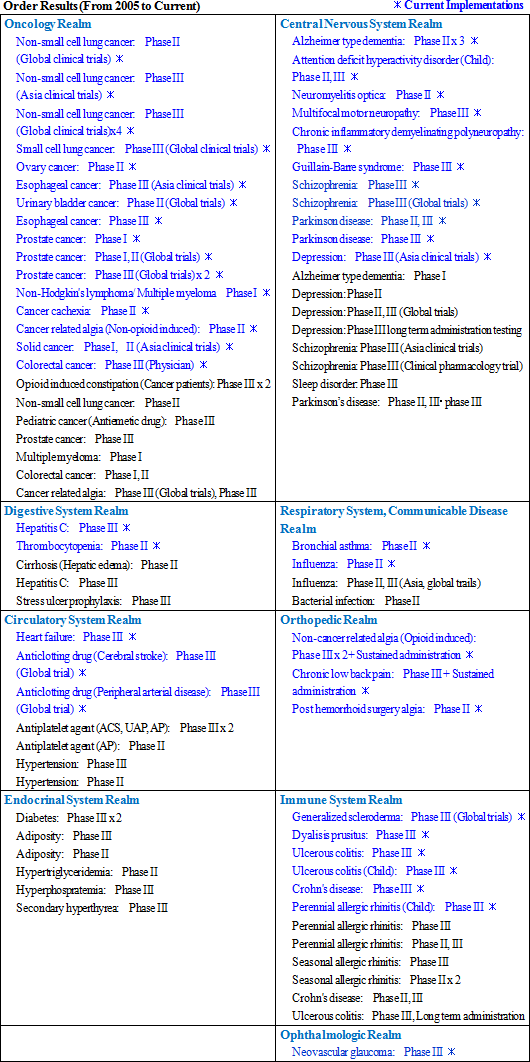 (3) High Profitability
The protocol deviation rates on projects undertaken by Linical have been held to extremely low levels, and the implementation period, including the period required for patient enrollment and data collection; for about 80% of all projects has been shortened. Because of its high levels of service quality and quick delivery times in highly difficult disease realms, Linical is able to book orders without having to offer reduced prices, and it is able to achieve high profit margins by overcoming economies of scale advantage of its larger competitors.Moreover, the source of Linical's earnings generation capability is its highly skilled and well-trained clinical research associates (CRA), as reflected in the good clinical practice (GCP) passport certification examination passage rates. The GCP passport certification examination is designed to promote improvements in the quality of Japan's clinical trials and clinical research and is conducted by the Japan Society of Clinical Trials and Research. Moreover, all of the employees eligible to obtain qualifications have gone through the examination process, and Linical achieved passage rates which are much higher than its competitors. 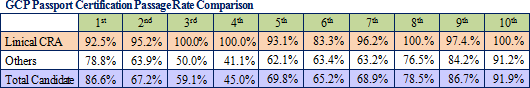 |
| Management Strategy |
|
(1) CRO Business
The main business strategy within the CRO business calls for 1) continued results to be achieved in the realms of oncology and central nervous systems, with repeat orders being converted into exclusive contracts, 2) early achievement of a structure with 300 CRAs within Japan to maintain high utilization rates of them, and 3) implementation of a global structure that enables one-stop contract order taking capability for jointly conducted global clinical trials.
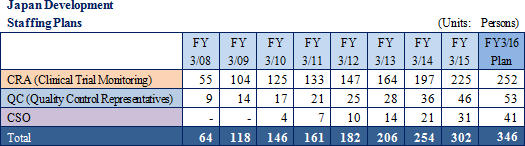 Global Development
Linical will leverage its global contract business structure for jointly conducted global clinical trials conducted in Asia, North America and Europe to accelerate the expansion in its global business. As part of the facilitation of a structure for clinical trials conducted between multiple countries, Linical Taiwan Co., Ltd. (Taipei City, Taiwan, capitalized at Taiwan $10 million) and Linical Korea Co., Ltd. (Seoul City, Korea, capitalized at 1 billion won) have been established as 100% owned subsidiaries. In April 2014, Linical Korea Co., Ltd. was merged with the Korean CRO service provider P-pro. Korea Co., Ltd. And in July 2008, Linical USA, Inc. was established in California to expand business in the United States and an office was established in San Diego, California in September 2014. Furthermore, Linical reached an agreement with Nuvisan Pharma Holding GmbH to acquire Nuvisan CDD Holding GmbH as a subsidiary to provide CRO services in Europe on October 29, 2014, with completion of the acquisition on December 1, 2014. At the same time, the company name was changed to Linical Europe GmbH. These developments have created a contract business structure in the three regions of Asia, North America and Europe.With the addition of the new consolidated subsidiary Linical Europe GmbH (Formerly known as Nuvisan CDD Germany GmbH) that provides CRO services in major countries in Europe, the Linical Group has significantly fortified and expanded its contract-undertaking structure and is now able to provides customers with comprehensive "global one-stop shopping" services. Linical Europe GmbH's subsidiaries are located in Germany, Spain, France, the Netherlands and Croatia, and monitoring services are offered to their neighboring countries. Consequently, the number of countries where the Linical Group offers its services has instantly been expanded to around 40 countries through its various partners. In addition, Linical Europe boasts of bountiful experiences and implementations of jointly conducted global clinical trials of major global pharmaceutical companies in the realm of oncology. Furthermore, strong synergies within the Linical Group are also expected to be derived. Moreover, Linical Europe also boasts of strong experiences in the data management responses including eCRF and CDISK, statistical analysis, and medical writing in addition to monitoring service for jointly conducted global clinical trials. Consequently, the Linical Group is expected to be able to acquire comprehensive orders for full services on jointly conducted global clinical trials. Also, Linical is considering expansion of its overseas facilities on the east coast of the United States (New Jersey and other states) where numerous Japanese and American pharmaceutical companies are located, and entering the South and Central American markets. With regards to Asia, to the Company has established offices in Singapore and now considering to enter markets in the Philippines China and Hong Kong. (2) Contract Medical Affairs Business
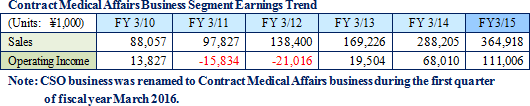
Linical differentiates itself from its competitors, which primarily offer only MR dispatch services, by focusing upon the contract service type drug development business. Specifically, the Company seeks to differentiate itself by hiring MRs with bountiful experience in certain patient realms and leveraging its bountiful knowhow established in the CRO business to take on highly specialized tasks. Currently, Linical provides the two main services of clinical research support and product marketing (liaison). With regards to the provision of contract support services for clinical research, the securing of quality levels of clinical research is a critical issue for evidence creation. Linical creates procedure manuals for structure facilitation support, monitoring and surveillance implementation. Cultivation of new medical institutions and physicians as clients in the process for market launch of new products in new realms, and execution of product differentiation strategies are conducted and proposed in the product marketing (liaison) service. In addition, the ability to receive work for clinical research was responsible for the turn to profits of this business segment during fiscal year March 2013, and new orders for clinical research and development in fiscal year March 2014 allowed sales and profits to rise from the previous term.
  (3) New Drug Discovery Support Business (New Business Development)
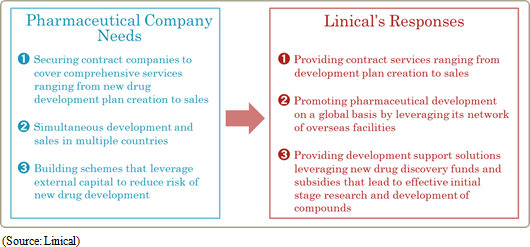
Linical is endeavoring to cultivate new drug discovery support services by responding to needs for comprehensive "one-stop shopping" services from the creation of development plans through application by providing new drug development schemes that have little impact upon near-term earnings. Demand for such services is growing at pharmaceutical and bio-venture companies. In addition, patients and the administrative authorities in Taiwan and Japan are encouraging companies to develop drug lag compound and new drugs in the realms of oncology, dementia, difficult diseases and regenerative medical treatments. Linical seeks to cultivate a new drug discovery support business that responds to these recent needs. Therefore, the Company will make efforts for clinical trial implementation planning creation, data collection and other tasks, and plan development of compounds in house at an early stage by leveraging subsidies and new drug discovery funds. Development of compounds using new drug funds is expected to help grow the Company's CRO business while allowing the experiences of the Company to also be leveraged. Also, experiences in undertaken tasks ranging from development planning to approval applications are expected to be gathered.
|
| First Half of Fiscal Year March 2016 Earnings Results |
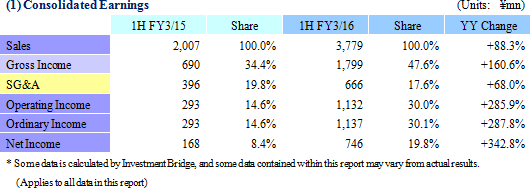 Sales, Ordinary Income Rise 88.3%, 287.8% Year-On-Year
Sales and ordinary income rose by 88.3% and 287.8% year-on-year to ¥3.779 and ¥1.137 billion, respectively. The markets for CRO and CSO services, in which Linical participates, is gradually expanding against the backdrop of increases in outsourcing of pharmaceutical product development and sales activities, and jointly conducted global clinical trials. Against this backdrop, sales of the CRO business grew by a large margin on the back of fortification of the global contract-undertaking structure in Japan, Asia, the United States, and Europe, favorable trends in orders for overseas projects and global joint clinical trials. The drug development business also saw higher sales due to the securing of new contract projects in the realm of post market launch clinical research.With regards to profit, increases in sales offset higher labor costs and a rise in amortization of goodwill and allowed profits to rise in the CRO business. Also, higher capacity utilization rates of staffing resulting from acquisition of new contract projects in post market launch clinical research contributed to results in the contract medical affairs business. Favorable sales in Taiwan and Korea, which had been expected to see losses but turned profitable during the second quarter of the current fiscal year, contributed an expansion in profits. Gross income margin increased by 13.2% points from the same term of the previous year to 47.6% during the first half, and sales, general and administrative expense to sales margin declined by 2.2% points year-on-year to 17.6%. Consequently, operating income increased by 285.9% year-on-year to ¥1.132 billion. Also, the booking of ¥16 million in foreign exchange translation gains in non-operating income allowed ordinary income to rise by 287.8% year-on-year. No other non-operating income/loss was recorded. 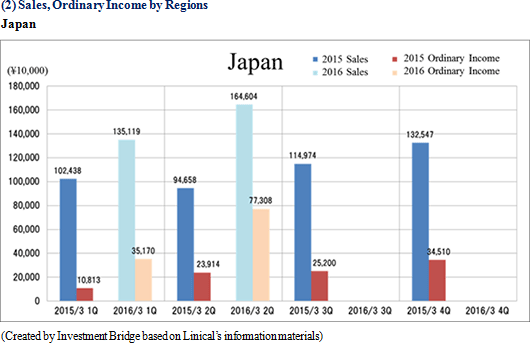 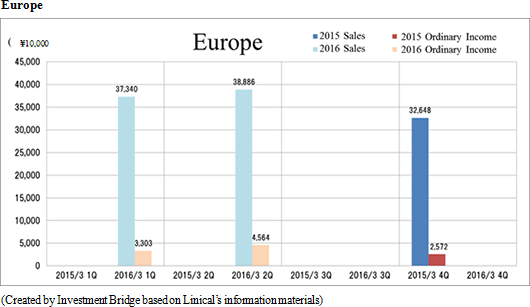 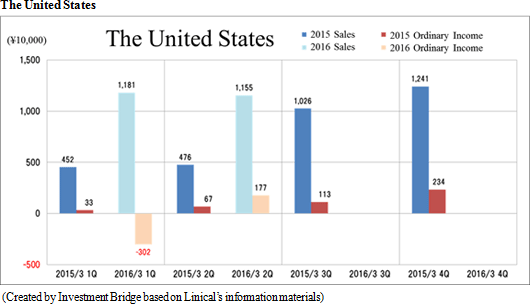 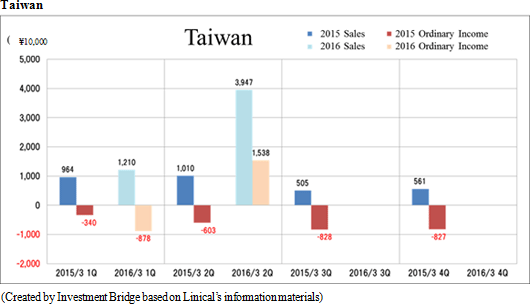 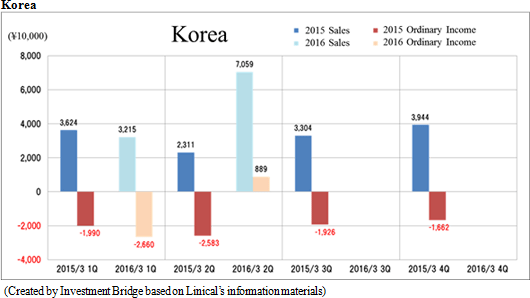 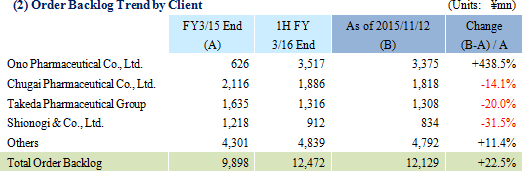 As of November 12, 2015, order backlog stood at levels 22.5% higher than those at the end of fiscal year March 2015. While the conversion of orders to sales of already formed contracts steadily progressed, this increase represents acquisition of new contract orders at a quicker pace than sales were booked. The growing trend for outsourcing and increase in jointly conducted global clinical trials contributed to a favorable order environment in the near term. Furthermore, successful marketing activities led to large numbers of contract work inquiries from both existing and new clients. Consequently, Linical maintains plans to fortify its contract-undertaking structure by further increasing the number of CRAs (Clinical research monitoring services). 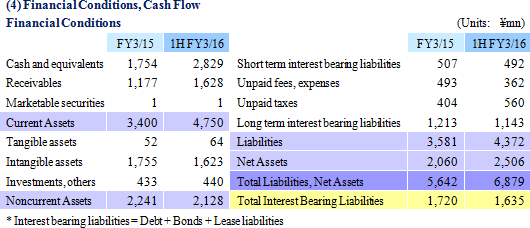 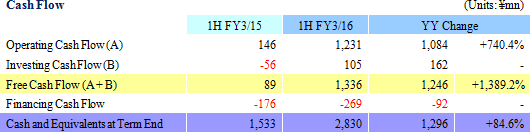 |
| Fiscal Year March 2016 Earnings Estimates |
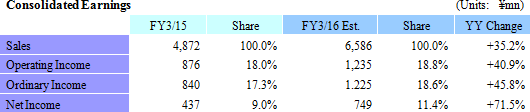 Sales, Ordinary Income Expected to Rise 35.2%, 45.8%
Linical's fiscal year March 2016 earnings estimates remain unchanged and call for sales and ordinary income to rise by 35.2% and 45.8% year-on-year to ¥6.586 and ¥1.225 billion, respectively. While the potential for an upward revision to earnings estimates exists in light of the fact that ordinary income during the first half exceeded 90% of the full year estimate, Linical maintained its earnings estimates due in part to its outlook for anticipatory expenses arising from aggressive overseas business expansion, potential for postponement or cancellation of some projects, and other factors in the normal progression of its business.Major pharmaceutical companies are attempting to offset the influence of major products that are going off patent by outsourcing tasks to rationalize and optimize operations, and by acquiring overseas venture capital companies to secure new developmental pharmaceutical products. Therefore, Linical can be expected to see an increase in the number of clinical trial contract projects it receives. In particular, seeing a lack of effective treatment methods in the realms of oncology and central nervous system diseases, the demand is extremely high for development of pharmaceutical treatment products. Against this backdrop and in addition to repeat orders based upon the high regard of customers for services in the CRO business, strengthened marketing activities for new customers are also expected to contribute to booking of new projects in the realms of oncology and central nervous systems and an expansion in sales. Moreover, the overseas subsidiaries in the United States, Korea and Taiwan, and subsidiaries in Europe newly acquired during the third quarter of the term just ended are expected to contribute largely to sales on a full-year basis. In addition, strengthening of marketing activities to new customers in the contract medical affair business is expected to contribute to an expansion in the customer base. The knowhow and expertise cultivated in the CRO business is expected to be leveraged to acquire new project orders in highly specialized realms. With regards to profits, both cost of sales, and sales, general and administrative expenses are expected to grow on the back of increases in the number of CRAs (Clinical development monitoring) and expansion of facilities in both Asia and North America. However, the higher sales and improvements in capacity utilization rates of CRAs are expected to contribute to a large margin of increase in profits compared with the previous term. Operating income is expected to rise by 40.9% year-on-year to ¥1.235 billion and operating income margin to rise by 0.8% points year-on-year to 18.8%. In addition, a decline in extraordinary loss resulting from a drop in retirement benefit obligations compared with the previous term is expected to allow net income to rise by 71.5% year-on-year. A 2-for-1 stock split is expected to be conducted to shareholders as of the registry date of January 1, 2016, and the adjusted dividend payment has been revised to ¥7 per share. However, this dividend payment remains in line with the previous term at ¥14 per share after the stock split is considered. |
| Conclusions |
|
At the same time, while ordinary income recorded during the first half achieved over 90% of the full year estimates, Linical refrained from revising its full-year estimates upwards. This decision to maintain existing estimates was made due to its outlook for anticipatory expenses for expansion of overseas business, potential for postponed or cancelled projects and other factors to be encountered in the course of the normal progression of its business. Given the few number of risk factors, Linical is expected to conduct a review of its earnings estimates based upon trends during the third quarter of the current fiscal year. The orders trends in the third quarter should be watched closely to determine whether or not the strong trends seen during the first half can continue throughout the remainder of the current term. Linical is also expected to aggressively expand the number of its staff in order to increase the speed of its growth on the back of the operations turning to profits in the United States and Taiwan during the second quarter. Important issues for Linical are its ability to increase staff numbers in the United States and Taiwan, where Linical is not widely recognized, and whether or not it can absorb the increased labor costs that this expansion plan entails without negatively impacting earnings. Disclaimer
This report is intended solely for information purposes, and is not intended as a solicitation to invest in the shares of this company. The information and opinions contained within this report are based on data made publicly available by the Company, and comes from sources that we judge to be reliable. However we cannot guarantee the accuracy or completeness of the data. This report is not a guarantee of the accuracy, completeness or validity of said information and or opinions, nor do we bear any responsibility for the same. All rights pertaining to this report belong to Investment Bridge Co., Ltd., which may change the contents thereof at any time without prior notice. All investment decisions are the responsibility of the individual and should be made only after proper consideration.Copyright(C) 2016 Investment Bridge Co., Ltd. All Rights Reserved. |



